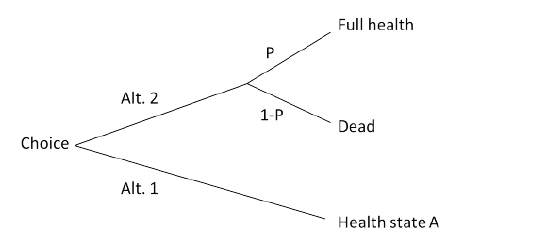
Figure 1: Standard Gamble, used to determine quality-adjusted life-year (QALY) weights


Thomas Davidson1,2* Sofia Tranæus2,3,4
1Division of Health Care Analysis, Department of Medical and Health Sciences, Linköping University, Linköping, Sweden*Corresponding author: Thomas Davidson, Division of Health Care Analysis, Department of Medical and Health Sciences, Linköping University, 581 83 Linköping, Sweden, Tel: +46 70 920 03 44; E-mail: Thomas.davidson@liu.se
Objective: Health economic evaluations provide decision makers with important information regarding the cost-effectiveness of technologies. However, such evaluations are still rare in most dental areas, and there is furthermore a need for methodological development in estimating costeffectiveness within dentistry. The purpose of this paper is to give an overview of methods used for estimating cost-effectiveness, and provide guidance for performing health economic evaluations within dentistry.
Methods: Available health economics methods are discussed and analysed according to their usefulness when assessing cost-effectiveness in dentistry.
Results: All types of health economic analyses may be suitable for evaluation in dentistry. It is most important that the outcome should be relevant to the decision problem. For this reason, various clinical outcomes are often used, such as DMFT or mm adjustment, number of infections, construction survival, etc. depending on what technology is assessed. It would be of value to also use quality-adjusted life-years (QALYs) as this is the most commonly used outcome measure in health economic evaluation, but this has rarely been done.
Conclusion: There is a need for more health economic evaluations within dentistry to be able to use scarce resources efficiently. In this paper we discuss methods for how this can be done, with a focus on the use of outcome measures relevant for decision makers.
Outcomes; Program evaluation; Economics; Cost-effectiveness
Direct treatment costs due to dental diseases worldwide have been estimated to represent 4.6% of global health expenditure [1]. Millions of dental procedures, prevention programmes, and treatments are being performed, even though the clinical evidence is often weak and information about their cost-effectiveness is rarely available [2,3]. In 2010, untreated dental caries in permanent teeth was the most prevalent condition worldwide, affecting 2.4 billion people, and untreated caries in deciduous teeth was the tenth most prevalent condition, affecting 621 million children worldwide [4].
Health economic evaluations provide decision makers with important information regarding the cost-effectiveness of technologies. This input is important when the aim is to use scarce resources efficiently. It is also of value for development of guidelines and funding of research. Even though a large part of oral health services are privately paid there is a need for guidance in cost-effective use of resources. Furthermore, many countries subsidize dentistry to some extent and it is important that public resources are used efficiently. In some countries, paediatric dentistry is subsidized to a high extent. For example, in Sweden, dental care is free until the year a person turns 19 years, and as of 2018 up to the age of 23. There may also be societal investments in public dental health programmes (such as caries preventive programmes), which require health economic evaluations. However, the vast majority of preventive measures are financed by the individuals themselves, and performed by them in the home setting.
Health economic evaluations are commonly used to decide what technologies should be reimbursed within health care [5] and the same considerations should apply to dental technologies as well. There may be specific challenges for technologies in dentistry, as discussed in this paper, but this is also the case for almost any other area within health care. In other words, these are not an adequate excuse for not evaluating costeffectiveness in dentistry. For example, in the last years there have been attempts to require that medical devices are proved to be cost-effective before they are reimbursed, even though this area is characterized by many challenges such as that the devices may be modified over time (due to technical advancement) and that the effects often depend on the training, competence and experience of the user [6]. The main reason why not more analyses of cost-effectiveness within dentistry are performed is probably because this is not explicitly requested.
The number of economic evaluation publications in dentistry is increasing and some systematic reviews have recently been performed. Tonmukayakul et al. [7] assessed 114 economic evaluation publications in dentistry and concluded that the methodological quality of such research has improved. Another systematic review, focusing on economic evaluations of caries prevention programmes, found that the “quality of the reporting needs to be improved” [8]. In addition, two recent studies discuss how to assess cost-effectiveness of certain areas in dentistry [9,10]. However, there is still a large need for guidance on the cost-effectiveness of technologies in most dental areas and, furthermore, for methodological development in estimating cost-effectiveness within dentistry. The purpose of this paper is to give an overview of methods used for estimating cost-effectiveness, and provide guidance for performing health economic evaluations within dentistry.
There are several types of health economic analyses. The main difference between them concerns how the outcomes are handled. Generally, all analyses search the opportunity cost of one technology in relation to the additional effect. The treatment in focus should be compared with the best alternative [11].
A cost-minimization analysis is concerned with costs only, and could be used when the consequences of the compared technologies are identical. By contrast, a cost-effectiveness analysis is used when the outcomes are expected to differ. The outcome measure in such an analysis could be any measure that is relevant for the treatment analysed, such as life-year gained, number of infections, prevented decayed-missing-filled teeth (DMFT), etc. A cost-utility analysis is similar to a cost-effectiveness analysis but relates outcomes to a utility index, normally quality-adjusted life-years (QALYs). A fourth type of analysis is the cost-benefit analysis, which uses monetary terms for both the costs and the outcomes. This is often measured by using willingness-to-pay measures. If the outcomes are valued higher than the costs in such an analysis, this means that the treatment has a positive net benefit at a societal level and, hence, that it should be implemented.
When a cost-effectiveness analysis or a cost-utility analysis is used the result provides a ratio between costs and effects for different treatments, called the “incremental cost-effectiveness ratio (ICER)”. To determine whether the ICER indicates a cost-effective option, the maximum willingness to pay for an effect needs to be known. For example, if societal willingness to pay for a QALY gained is €30,000, all technologies with an ICER below this value are assumed to be cost-effective at a societal level.
The analyses presented above can be complemented by a budget impact analysis [12] evaluating how one or several budgets are affected by the introduction of a new technology, and what other consequences are expected for the main actors.
All types of health economic analyses may be suitable for evaluation in dentistry. The analysis can be performed using different perspectives, of which the societal one is the broadest, including all costs and effects in society, no matter for whom. Other commonly used perspectives concern health care, and the clinic’s and patient’s perspective. There is no agreement on what perspective to use5 ; it depends on what the analysis aims to provide information about. The different perspectives affect both costs and outcomes, but are most often discussed for costs only.
All methodological guidelines in health economic evaluations are in agreement that analysis of costs and effects should be based on a time horizon that is sufficiently long to reflect all important differences in costs or outcomes and that is often determined using a decision analytic model [5]. This means that evaluations in prosthodontics, for example, need to include all future costs and effects related to the treatment, which probably would be a lifetime evaluation. The same goes for caries preventive treatments, as this may affect the future incidence of new caries. Costs and effects that occur in the future should be discounted annually to reflect their values at the time the analysis is undertaken. The discount rates may vary between guidelines [5]. The two main parameters in the estimation of cost-effectiveness concern, obviously, “costs” and “effects”, and these parameters will therefore be presented in greater depth below.
The cost of a technology is more than its price, in terms of money, time, staff, etc. Costs are caused by resources used and should be valued based on what the resource could otherwise have been used for (i.e. the opportunity cost). Preferably, all resources used should be quantified and presented before they are valued, to enable transparency and transferability to other settings.
Costs could be classified in several ways. In a recent paper by Bassi et al. [9], costs are presented in terms of four main areas:
“Indirect costs in initial treatment” is defined as professional time associated with maintenance events and clinic overhead costs. By “indirect patient costs” is meant the patient’s time and other expenses (such as travel costs, parking). Maintenance costs consist of all treatment costs that are not part of the initial treatment.
To capture the full direct costs, the number of minutes the dental profession spend on the patients are measured and valued according to the total cost of the time used. All time used by patients (and relatives and others, if relevant) should also be included if a societal perspective is aimed for. If the technology requires repeated visits to the dental clinic, all time used needs to be aggregated. The value of this time may be difficult to estimate but it should represent the opportunity cost. If the time used by the patients would otherwise have been spent on paid production, it is the cost of having a person employed that should be used (the human capital approach). If leisure time is used, the opportunity cost of this leisure time should be used.
As stated in section 2, in a health economic evaluation, any outcome measure may be used. It is most important that the outcome should be relevant to the decision problem. For this reason, various clinical outcomes are often used, such as DMFT or mm adjustment, number of infections, construction survival, etc. depending on what technology is assessed. However, the value of these outcomes is not known, nor how important they are assumed to be by patients, which leads to the conclusion that it is hard to use such outcomes to reach an optimum of resources used. For example, if an analysis presents that it would cost €500 per prevented DMFT in a caries preventive programme, would that be assumed to be cost-effective or not? Furthermore, when different outcome measures are being used it is hard to draw a comparison between different analyses.
The purpose of most dental technologies is to improve individuals’ quality of life (QoL). More specifically, it is the aspects concerning oral health that are aimed to be improved – in other words, oral healthrelated QoL (OHRQoL) [13]. When measuring QoL, health-related QoL (HRQoL) or OHRQoL, the measuring instrument needs to be chosen, as well as the time and frequency of measurement [14]. The instruments could roughly be divided into generic and specific measures. Examples of generic measures are the 36-item short-form health survey (SF-36) and EuroQol’s five-dimension (EQ-5D) instruments. The most used specific measure in dentistry is the Oral Health Impact Profile (OHIP) [15]. The original version of the OHIP comprised 49 items divided into seven domains, but shorter versions (14 and five items) of the instrument are also available [16,17]. Another measure is the Geriatric Oral Health Assessment Index (GOHAI) [18]. There have also been suggestions to use a measure called “quality-adjusted tooth years (QATYs)” [19] to represent individuals’ OHRQoL, but this has not been widely used.
The most commonly used outcome measure in health economic evaluations is quality-adjusted life-years (QALYs), which combines a value of the HRQoL of a health state (QALY weight) with the time of that health state. Quality-adjusted life-years are also expected to represent individuals’ preferences for health, but the measure has shortcomings [20] and should rather be seemed as a measure of health. So far, QALYs have not been extensively used in dentistry.
There are several methods for eliciting QALY weights, as presented below and as also discussed in some systematic reviews concerning preference-based outcome measures in dentistry [21,22]. Methods for estimating QALY weights can be divided into direct and indirect methods. The direct methods commonly used are Standard Gamble [23], time trade-off (TTO) [24] and the rating scale method.
The Standard Gamble approach estimates the value of a health state by finding a probability (P) in which an individual is indifferent between living in that health state, and participating in a gamble with a P of living with full health, but with a risk (1-P) of immediate death (Figure 1).

Figure 1: Standard Gamble, used to determine quality-adjusted life-year (QALY) weights
The Standard Gamble technique has been used in some attempts to estimate QALY weights in dentistry [25-27]. Two of these [26,27] did not include risk of death in the gamble and their values can therefore not be interpreted as true QALY weights (as long as no transformation is undertaken).
The TTO method is used to elicit the number of years an individual is indifferent between living in a certain health state and living in full health. If the individual states that living 10 years in health state A is equal to living 5 years in full health, then the QALY weight of health state A is 0.5 (Figure 2).
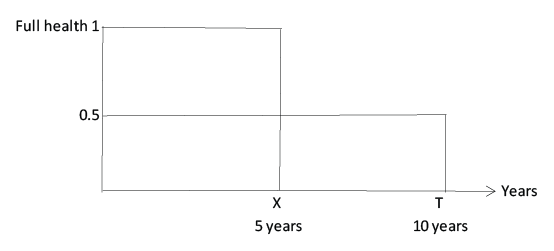
Figure 2: Time trade-off (TTO), used to determine quality-adjusted lifeyear (QALY) weights
In some studies that use TTO in dentistry [28,29], number of years has been replaced with sacrifice of free time or time with dental health before treatment and, as in some of the examples presented above, this does not provide valid QALY weights. One study where this method has been used in a way that enables calculation of QALY was performed by Cunningham et al. [30]. By using the TTO approach, five times each for 21 patients undergoing orthognathic treatment, they found a decrease in QALY weights during the treatment procedure, but an increase after the treatment was complete. Overall, the total QALY gain was high (extending the analysis to a lifetime perspective).
Using a rating scale to estimate QALY weights is easier than the methods presented above, but the theoretical foundation of this method is weaker. Using the rating scale, individuals evaluate health states by ranking them on a cardinal scale that is normally anchored between “best imaginable health” and “worst imaginable health” (Figure 3). The main theoretical problem is that when individuals do not have to make a choice between two alternatives their true preferences are not revealed.

Figure 3: Rating scale for estimating quality-adjusted life-year (QALY) weights
Some studies have used the rating scale to assess preference outcomes in dentistry. For example, Nassani and Kay [31] measured values in relation to tooth loss, Cunningham and Hunt [32] estimated values for dento-facial deformity and Fukai et al. [33] valued health states in order to compare the outcomes of an educational intervention in the field of dental health.
Cunningham and Hunt [32] also compared QALY weights elicited by different direct methods, and furthermore compared values elicited by patients and by a general public. All methods used in their study included “dead” as an anchoring point. They found no differences between the utility values for the two groups of respondents, but the various methods gave different results. The highest values were found using Standard Gamble, 0.85, while the lowest were found using a rating scale, 0.57. The value elicited by TTO was 0.75. All the methods used were found to be acceptable to respondents.
Indirect methods to elicit QALY weights are based on questionnaires with a pre-scored value set derived by one or several of the direct methods (using a multi-attribute utility measure). For example, the EQ-5D instrument consists of a questionnaire with five questions. Each combination of responses to these questions can be assigned an HRQoL weight using specific value sets. The British value set, which is commonly used, has been developed by using TTO and the RS in a sample of the British general public [34], but today many value setsare available. Other questionnaires that can be used to indirectly elicit QALY weights include the short-form six-dimensions (SF-6D) [35], Health Utilities Index (HUI) [36] and Child Health Utility Index 9D (CHU9D) [37]. As far as we know, only the EQ-5D [38] and CHU9D [39] have been used in dentistry, but none of them seem to be sensitive to changes in dental health.
It would be valuable if QALY weights could be elicited from the OHIP, but no such method has yet been developed. However, Brennan and Spencer have mapped results from the OHIP-14 to the EQ-5D in order to elicit QALY weights from the OHIP-14 [40], and conclude that this enables health state values to be derived from OHIP-14 scores.
Individuals’ willingness to pay for a treatment would theoretically equal their preferences for the treatment (and for the related health state), but the willingness-to-pay method is nevertheless often difficult to use. It has been applied in several dentistry studies with the aim to find the strength of preferences for dental health [41-43], but all attempts have potential risks of biases. Furthermore, individuals’ willingness to pay is generally related to their ability to pay, which means that this method may be in conflict with ethical principles guiding decision making.
The results should be presented as an ICER as long as none of the alternatives being compared is dominant, i.e. has lower costs and better effects than its alternative (s). Presentation of the ICER should also be complemented with the absolute costs and effects of each alternative strategy. This is important in order to assess whether a result can also be assumed to be clinically relevant.
The ICER can be graphically presented in a cost-effectiveness plane in which two technologies are compared (Figure 4). New technologies are often located in quadrant B in which the assessed technology leads to higher costs and improved effects compared with the alternative technology. If this estimate is below the willingness-to-pay threshold, the technology is considered cost-effective. However, in most countries there is no explicit such threshold.
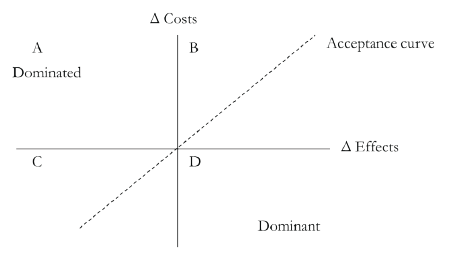
Figure 4: The cost-effectiveness plane. One technology is compared with another and incremental costs and effects are illustrated in this plane. For example, if the assessed technology costs more than the comparator, the ICER will be located on the upper part of the Δ Costs axis, and if it improves effects it will be located on the right side of the Δ Effects axis.
The uncertainty surrounding the results should be explored in sensitivity analyses, preferably using both deterministic and probabilistic sensitivity analysis [44]. The uncertainty could also be displayed in the cost-effectiveness plane.
There is a need for more health economic evaluations within dentistry to be able to use scarce resources efficiently. In this paper we have discussed methods for how this can be done, with a focus on the use of outcome measures relevant for decision makers. Health economic evaluations could furthermore help in preventing socio-economic inequalities in dental health and support research in preventive dental care. The most commonly used types of health economic evaluations have been discussed, and cost-effectiveness analysis (including cost-utility analysis) has been presented as the preferred method.
Before choosing methodology, the decision problem needs to be clearly defined. Generally this means defining the patients, intervention, comparator and outcomes (PICO), but the decision also concerns aspects such as the perspective of the analysis (i.e. the perspective of the decision maker) and what cost-effectiveness threshold should be used. At a societal level, it is the societal willingness to pay for health that sets the threshold for cost-effectiveness, but if a more restricted budget is used the threshold may be set at another level. As dental care is often financed privately the relevant question of cost-effectiveness is then a matter for the individuals. If, however, technologies are to be subsidized from public funds, it would be necessary to analyse the societal willingness to pay. Furthermore, guidelines may use a broad perspective to strive for a societal optimum even if individuals pay themselves. In such a situation the individuals can be guided in their decision making, but take the final decision themselves, concerning whether they find the technology cost-effective or not in relation to their own willingness to pay. In this respect, it is of highest importance to assess values that are really important to the patients. Dental professionals might focus on “aesthetics” while patients point out the social function of aesthetics, for example, “kissing”. Similarly, the chewing function may be central to professional considerations while patients may express this as a social function, viz “meal joy” [45].
It is generally recommended to use checklists to make sure all important aspects are dealt with in the analysis [11]. However, these checklists need to be thought of in relation to their influence on the decision problem and not in relation to the percentage of boxes that are ticked. Even if some parts of the checklist are left unanswered, this does not necessarily mean that there is a problem with decision making. Issues such as the type of evaluation method is not described or no discount rates have been used may not be a problem in many cases, while important aspects outside the checklist could be missed, for example: is the analysis relevant for the decision problem; is the best comparator included; and has the most relevant outcome measure been selected?
To provide dentistry with relevant health economic evaluations, there is furthermore a need for well-developed simulation models that can analyse technologies over a relevant time horizon, combining sources from different areas, and a need to explore total uncertainty. Furthermore, health economic evaluations in dentistry would also benefit from estimating QALY weights for various dental health states, preferably ones that are related to states given by the OHIP.
In this paper we have touched upon some important areas in assessing the cost-effectiveness of dental technologies, but this picture is far from complete. It is time to assess cost-effectiveness of technologies in dentistry.
Download Provisional PDF Here
Article Type: Research Article
Citation: Davidson T, Tranæus S (2016) Time to Assess Cost-Effectiveness of Technologies in Dentistry. Int J Dent Oral Health 2(5): doi http:// dx.doi.org/10.16966/2378-7090.200
Copyright: © 2016 Davidson T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access