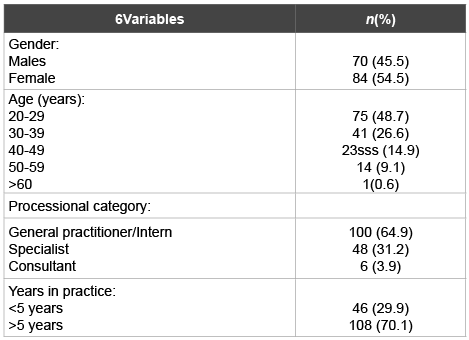
Table 1: Demographic and professional characteristics of practicing dental practitioners

Juma Alkhabuli1* Mawlood Kowash2 Aqsa Shah3
1Basic Dental Sciences, RAK College of Dental Sciences, Albrirat, Ras Alkhaimah, RAK, UAE*Corresponding author: Juma Alkhabuli, Basic Dental Sciences, RAK College of Dental Sciences, Albrirat, Ras Alkhaimah, P.O.Box 12973, RAK, UAE, Tel:+97172222593/2269; E-mail: juma@rakmhsu.ac.ae
Emergence of bacterial strains resistant to antibiotics has been a major concern to medical and dental profession. Irrational antibiotic prescription by dental professionals is well documented. The aim of this study was to explore the knowledge and attitude of dental practitioners (DP) working in northern emirates of UAE towards antibiotic prescription and resistance development.
Methods: A questionnaire was distributed to 200 DP working in Northern emirates dental clinics. The questionnaire sought answers to clinical and non-clinical conditions for which antibiotic would be prescribed and the potential contributing factors in development of antibiotic resistance.
Results: Out of the 200 questionnaires sent out154 (77%) responded. 54.5% were females. The majority of DP would prescribe antibiotics for elevated temperature (87.7%), diffuse swelling (94.1%) and swelling causing eye closure (83.1%) conditions. Antibiotic prescription would be considered for pericoronitis, cellulitis and trismus by 76.0%, 90.9% and 47.4% of the DP respectively. However, 53.3% would prescribe antibiotics for fluctuant localized swelling, 72.5% to reduce postoperative complications and 67.8% for surgical extraction procedures.
Generally, amoxicillin was the most preferred drug. 44.6% of the DP preferred augmentin in treatment of cellulitis. DP with less than 5 years in practice showed higher mean knowledge with regard to antibiotic indications (p=0.039). 70.8% thought widespread use of antibiotics was an important factor in development of antibiotic resistance.
Conclusion: In spite of the DP fair knowledge, a considerable percentage continued to prescribe antibiotics irrationally. The study highlights on the importance of promoting knowledge on antibiotics use and abuse through continuous dental education forum.
Antimicrobial; Odontogenic infection; Antibiotic abuse; Resistant strains; Dental practice
Antibiotic therapy has been in use for more than 80 years playing major role in treatment and control of infectious diseases. The safe use of systemic antibiotics has undoubtedly improved the quality of life and increased life expectancy for millions of people worldwide.
Despite the indispensable benefits of systemic antibiotics, there has been an explosion in the number of bacteria that have become resistant to several drugs in use. In fact not the antibiotics per se is the culprit, as they remain one of the most potent weapons against diseases caused by microbial infection, but the inappropriate use of the antibiotics resulted in disastrous situation due to bacterial mutations developing resistant strains.
It is well known that several strains of bacteria have demonstrated resistance to a wide range of medically crucial antibacterial drugs. Probably, methicillin-resistant Staphylococcus aureus has become one of the most frequent hospital acquired pathogen. The swift development of resistance to antibacterial drugs is worrying and has contributed significantly to hospital cost, morbidity and mortality [1].
The oral microbial flora is comprised of diverse range of microorganisms including bacteria, fungi and protozoa but only a small percentage can be isolated by the conventional culture technique. Recently, use of advanced molecular biological methods revealed several novel phylotypes that cannot be recognized by conventional techniques [2].
The best way to win a battle is to thoroughly understand the enemy. Thus the rational choice and use of antimicrobial agents begins with the knowledge of the microorganisms most likely responsible for common dental infections.
Among bacteria that are potentially pathogenic and occasionally are found in the oral cavity include Staphylococcus aureus, Enterococcus faecalis, S. pneumoniae, Streptococcus pyogenes, Neisseria meningitidis, members of the family Enterobacteriaceae, Haemophilus influenzae and Actinomycetes [3]. Furthermore, the oral microbial flora is dynamic and changes constantly throughout life. Several commensal microorganisms can cause diseases in a suitable environment.
In dental practice antibiotic therapy is mainly used to treat or prevent spread of odontogenic infection. Other contentious uses include prophylaxis for patients with joint replacement, infective endocarditis and conditions associated with systemic diseases, such as diabetes mellitus. Thus, the dental conditions that require use of systemic antibiotics remain limited and the majority of dental emergencies, including cases with acute dental pain can be managed by local intervention [4]. Presence of severe pain, such as acute pulpit is for example, is not a justification for antibiotic therapy; rather its use is reserved for conditions associated with evidence of systemic spread [5].
There is over whelming evidence in literature that the dental professionals have contributed to antibiotic misuse and development of bacterial resistance [6-10]. Many authors have extensively investigated the numerous factors related to improper prescription of antibiotics including but not limited to uncertainty or failure of making definite diagnosis, lack of knowledge of adverse reactions, over-prescription, self-medication, and lack of time for immediate treatment (convenience) or inability to find out the causative agent [11-15].
The main motive that prompted us to carry out this preliminary study is the increasing number of referred patients from private dental clinics and dental centers to Ras Al Kkaimah (RAK) College of Dental Sciences (RAKCODS) clinics seeking treatment after failure of drug therapy. In many of the referred cases, antibiotic therapy was not based on sound clinical principles and current guidelines. Cases of reversible and irreversible pulpit are which need immediate mechanical intervention were put on broad spectrum antibiotics for long periods. Frequent and indiscriminate use of antimicrobial drugs and potential development of resistant strains of oral bacteria is truly alarming. The broad spectrum antibiotics, which some of dental practitioners use it as “pain killer” are precious and required for life-threatening conditions, when no alternative remedies would be present.
It has been reported that in some countries up to 84% of the dentists have tendency to prescribe antibiotics in absence of clinical indications [16]. This worrying figure warrants investigation to limit antibiotic abuse. Unfortunately, not all dentists are acquainted with the most current clinical guidelines regarding antibiotic prophylaxis, albeit their availability. This could be the reason for the empirical prescription of antibiotics and subsequent development of adverse consequences [17]. Furthermore, the clinicians are unaware of the microorganisms responsible for the infection because cultures are not commonly grown from patient’s abscess or exudate and the treatment is relied on the pre-available epidemiological data of the potential causative bacteria.
Data available from previous studies reveals that the dental practitioners tend to prescribe antibiotics for longer period than required, yet imprecise dose [10,14,16]. Nevertheless, the same data sources assert that the dentists have adequate knowledge and aware of the antibiotics use guidelines!
Salako et al. [14] reported a large number of dentists would prescribe antibiotics until they find time to perform definitive treatment. This empirical antibiotic therapy is unethical and its disadvantages supersede the benefits.
Self-medication is another emerging factor in development of antibiotic resistance and should not be underestimated. In United Arab Emirates (UAE), practice of self-medication is not uncommon. In one of the studies involving University students, the frequency of antibiotics use without prescription was 40% [18] and 56% among community people [19].
In developed countries not a single dose of antibiotics can be obtained without prescription, while in developing countries, including the Middle East, a part from narcotics, most of the drugs including antibiotics can easily be obtained without prescription from any community pharmacies. Notwithstanding, the UAE’s antimicrobial policy restricts dispensation of antibiotics without prescription, antibiotics are still freely available over the counter [19].
The information available about the rationale behind the use of antibiotics by the practicing dental surgeons in UAE is scarce. The objectives of this study were, therefore to explore the knowledge and attitude of the dental surgeons practicing in the UAE northern emirates towards antibiotic therapy and its resistance.
This is a descriptive, cross sectional, questionnaire based study. The research has been approved by the research and ethics committee of RAK Medical and Health Sciences University, UAE.
The self-administered, structured questionnaire was a modification of that described by Palmer et al. [20]. To ensure validity of the questionnaire, a pilot study was carried out and discussed with a group of colleagues and modified accordingly.
Two hundred printed questionnaires were distributed randomly to dental practitioners working in the five northern emirates of UAE, namely Sharjah, Ajman, Ras Al-Khaimah, Fujairah and Um Alquwain. The study sample included general dental practitioners (GDPs), specialists and consultants working in private sectors, government hospitals and dental centers. The questionnaires were handed over to each sector and distributed to all working practitioners to complete and to be recollected after 1 week.
In addition to demographic information, the questionnaire was comprised of 7 sections. Dental practitioners were asked to provide definite answers to clinical and non-clinical parameters including symptoms and treatment modalities related to their patients, which govern dental practitioner’s decision of prescribing antibiotics.
Among the instructions included in the questionnaire is not to use any external resources while responding to the stated questions. Demographic information including age, gender, professional qualification and years in practice were sought.
We also sought how a practitioner would assess clinical signs and symptoms, such as elevated temperature, presence of localized fluctuant swelling, gross diffuse swelling, mouth opening restriction, difficulty in swallowing and closure of eyes due to swelling in prescribing antibiotics.
Since dental practitioners may prescribe antibiotics for causes other than infection, factors for which antibiotic therapy was considered, such as convenience, social background, delay of treatment, prevention of post-operative complications and uncertainty of diagnosis were therefore explored.
In addition, participants were required to provide their judgments as whether to prescribe antibiotics or not for specific clinical conditions, such as acute and chronic pulp diseases related to dental caries, periodontal abscesses and gingival diseases, extraction and related surgery, tooth replantation and trismus.
The questionnaire also investigated the preferred antibiotics a dental practitioner would use in case of certain conditions including periapical infection, pericoronitis, cellulitis, apicectomy, trismus and other dental infections. The suggested antibiotics were Amoxicillin, Augmentin, Erythromycin, Metronidazole, Tetracycline and Cephalosporin. In addition, the participants were asked to provide their opinions with regard to factors contributing to development of antibiotics resistance. Factors such as use of broad spectrum antibiotics, poor access to culture and sensitivity, wide use of antibiotics, inappropriate duration, lack of guidelines and patients’ demand and expectations were investigated.
Evaluation of participants’ knowledge on recommendation of antibiotic therapy for the 22 listed conditions was based on guidelines published in certain literatures [21-24]. For each correct answer grade (1) was given versus grade (0) for the wrong answer. Therefore, the total theoretical knowledge range was from 0 to 22.
All descriptive data were projected as frequencies and percentages and compared using chi-squared test, while quantitative data were presented as means and standard deviations (SD) and compared using t- test.
The level of statistical significance of all tests was 2-tailed P-value <0.05. All tests were performed using SPPS version 16.
Out of the 200 questionnaires sent to the practitioners, 154 (77%) forms were returned.70 (45.5%) of the respondents were males and 84 (54.5%) were females. Table 1 shows the demographic and professional characteristics of the respondents. Among the participants, more than half (64.9%) were general practitioners or interns. The consultants were the minority (3.9%). The majority of the participants (70.1%) practiced dentistry more than 5 years.

Table 1: Demographic and professional characteristics of practicing dental practitioners
Table 2 shows the common antibiotic prescription patterns of dental practitioners working in the northern emirates according to clinical symptoms and general considerations. The majority of the dental practitioners would prescribe antibiotics if there is clinical evidence of temperature elevation (87.7%), diffuse swelling (94.1%) or swelling causing eye closure (83.1%). 53.3% of the respondents would still prescribe antibiotics for fluctuant localized swelling. A substantial percentage of dental practitioners (72.5%) would prescribe antibiotics postoperatively to halt potential complications. If the diagnosis is non-conclusive or a decision to postpone the treatment is taken, 16.6% and 31.1% of the respondents would prescribe antibiotics for their patients respectively. However, in cases presented with difficulty in swallowing, 47.0% of the respondent dental practitioners would consider antibiotic therapy.
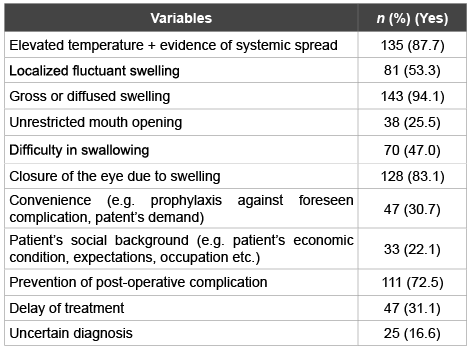
Table 2: Antibiotic prescription patterns among dental practitioners for selected clinical signs, symptoms and general considerations
The questionnaire has also reviewed the antibiotic prescription consideration of the dental practitioners for clinically diagnosed conditions (Table 3). The table demonstrates a wide range of variation among the respondents. Antibiotic prescription would be considered for cases diagnosed with pericoronitis, cellulitis and trismus by 76.0%, 90.9% and 47.4% of the respondents respectively. A considerable percentage of the respondents (67.8%) would prescribe antibiotics for surgical extraction, while 17.5% would consider the same for routine extraction. 39.6% of the dental practitioners would prescribe antibiotics for dry sockets. Acute pulpit is and acute periapical infection conditions were also considered for antibiotic therapy by 26.3% and 63.6% of the respondents respectively. Antibiotic prescription for sinusitis as a result of dental infection was also investigated and a significant percentage of dental practitioners (74.0%) would consider prescription of antibiotics. Up to 66.2% of the dental practitioner would prescribe antibiotics for periodontal abscess. When root canal treatment is considered, 71.9% of the respondents would recommend antibiotic therapy for apicectomy procedure. Generally, 24.7%of the respondents would prescribe antibiotics for root canal surgery pre-operatively and 40.5% post-operatively.
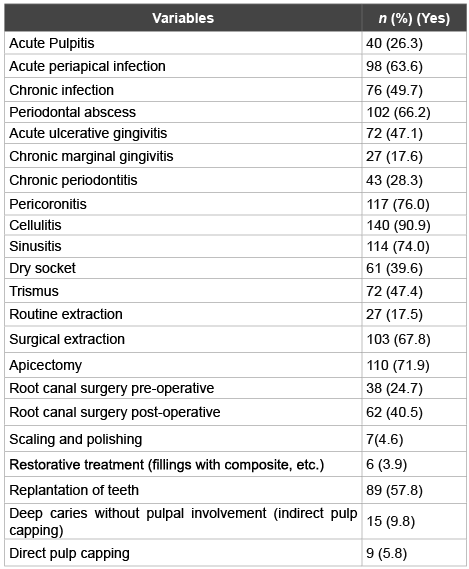
Table 3: Clinically diagnosed conditions for which dental practitioners would prescribe antibiotics
149 (98.0%) of the respondents are aware of the antibiotic resistance problem. Also, 124 (80.5%) of the respondents claimed that they assess the significance of antibiotics in their practices.
Table 4 shows the antibiotic preferences among the dental practitioners for selected clinical conditions. A part from treatment of cellulitis, Amoxicillin was the most preferred antibiotics for the selected conditions. If the patients are not allergic to penicillin, Amoxicillin would be prescribed for periapical infections by 51.9% of the respondents, dental infection by49.3%, pericoronitis by 38.0% and apicectomy by 52.1% of the respondents. In treatment of cellulitis, 44.6% of the respondents would prescribe Augmentin compared to Amoxicillin (29.1%). A significant antibiotic commonly used for anaerobic infection, Metronidazole would be prescribed for pericoronitis by 12.7%, cellulitis 2.7%, trismus 5.8%, dental infection 4.7% and in apicectomy by 6.2% of the respondents. The data shows that Tetracycline and Cephalosporin were barely prescribed by the respondents. In certain conditions, more than one antibiotic might be prescribed by the dental practitioners. These conditions include periapical infection by 13.6%, cellulitis 14.9%, trismus 11.5% and dental infection by 8.7% of the respondents.
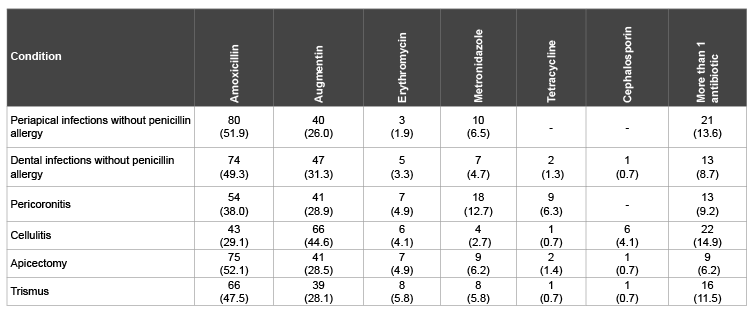
Table 4: Preferred antibiotics for selected clinical conditions by dental practitioners, n (%)
The survey has explored the dental practitioners’ assessment of the contributing factors to development of the antibiotic resistance (Figure 1). 70.8% of the dental practitioners thought widespread use of antibiotics is a very important factor contributing to antibiotic resistance. Inappropriate duration of the antibiotic course and lack of prescribing guidelines were rated 2nd and 3rd respectively among the very important factors in development of antibiotic resistance. Also, poor updates on antibiotic resistance, use of broad spectrum antibiotics and poor access to culture and sensitivity test were considered as important factors in development of antibiotics resistance by 52.9%, 47.4%, 47.1% of the respondents respectively. Patients’ demand and antibiotics promotion by pharmaceutical companies were measured as important factors in antibiotics resistance by 40.9% and 45.1% of the dental practitioners respectively.
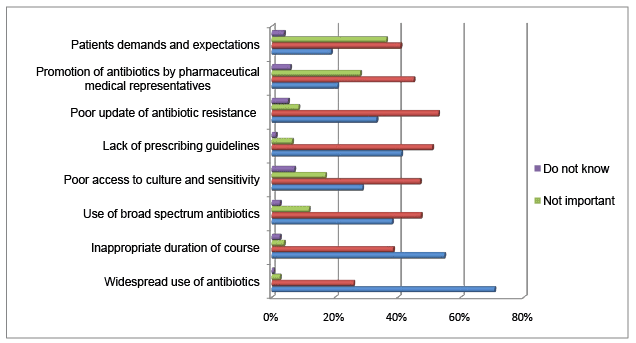
Figure 1: Percentages of dental practitioners’ assessment of factors contributing to antibiotic resistance
Knowledge of the respondents with regard to response to the use of antibiotics for clinically diagnosed conditions (Table 3) was formulated as described in the methods section. The mean of the Knowledge score for all respondents for the 22 conditions was 14.83 (SD 0.45) (in a possible range of 0-22). There were no statistically significant differences in means of knowledge by the gender, age and professional ranks. Only years in practice demonstrated some statistically significant difference. Practitioners in less than 5 years of practice appeared to have a higher mean knowledge on indications of antibiotic use than senior practitioners (p=0.039), (Table 5). On an average, the knowledge percentage among all respondents is about 67%, which is considered fair.
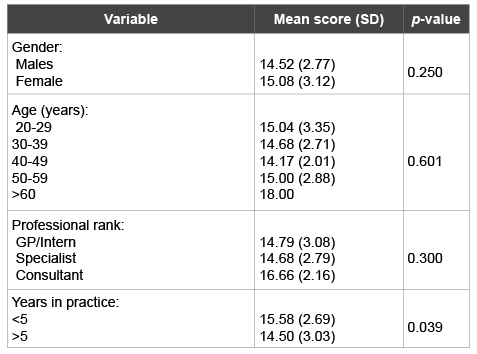
Table 5: Knowledge of the dental practitioners about the appropriate use of antibiotics by gender, age range, years in practice and professional rank variables
The numbers and percentages which are given in the tables represent the number of the dental professionals who actually responded to the questions.
Searching the PubMed and other data sources using keywords; knowledge, attitude, prescription, dentists, dental practitioners, antibiotic and resistance in UAE revealed no results. However, a report [25] explored similar characteristics among dental practitioners working in Iran, Jordan and UAE. Our study, therefore involved the dental surgeons practicing in the UAE (northern emirates). A 77% response rate is considered good response. More than half of the surveyed candidates were GP/Interns, forming the main working dental force in the community.
In principles, any dental infection needs treatment by proper elimination of source of infection, incision and drainage of abscess if present. Antibiotics work as adjunct but cannot substitute local intervention and are invariably prescribed on assessment of the clinical signs and symptoms.
Elevated temperature is a reaction of host defense and immune response mechanisms for competing infection. Presence of fever therefore is an absolute indication for antibiotic therapy. Nearly 88% of the surveyed dental practitioners would use antibiotics for patients with elevated temperature. Previous regional studies reported similar figures [14,25,26]. Facial cellulitis, a diffuse swelling commonly associated with periapical infection spread that may extend beyond mid face causing eye closure is another condition which necessitates antibiotic cover. It is appreciated to see a large percentage (94.1%) of the respondents is acquainted with the seriousness of the ailment and its antibiotics requirement. In fact, the previous two conditions represent fundamental bases of infection, infection spread and its sequel, which are known to all medical professionals. Therefore, lower percentages of agreement would probably unacceptable.
On the other hand, it is worrying to see considerable percentages of practicing dentists would still prescribe antibiotics if they were uncertain of the diagnosis or on patients’ demand. This trend of antibiotic abuse is not uncommon and well reported [14,25]. Although various excuses were put forward to explain such practice, such as dentists working in crowded clinics or lack of time, the dental practitioners need to bear in mind of the deleterious effects on long run.
It appears that management of localized fluctuant swelling is still confusing among dental practitioners including specialists. Half of the respondents tend to prescribe antibiotics, while local intervention is primarily what is needed for such conditions. Additionally, no significant differences were found between general practitioners and specialists’ approach (p=0.031, Chi-Square test). It is worth noticing that the specialists included in the survey were not identified as oral surgeons or other specialty.
Difficulty in swallowing and mouth opening restriction both are invariably signs of fascial spaces infection spread and need not to be taken lightly. A considerable percentage (47.0%) would prescribe antibiotics for cases associated with difficulty in swallowing. In other regions of the world, probably the dental surgeons are more aware of these conditions thus higher percentages were noticed [27]. It is well known that the fascial spaces are poorly vascularized and antibiotics may not reach the deepest part of the infection. Therefore, these conditions require thorough surgical intervention, as antibiotics therapy alone might not be enough to clear the infection.
The dental practitioners seem to less keen to prescribe antibiotics if they are not certain of the diagnosis (16.6%), (Table 2). This is a good sign when compared to responses of dental practitioners practicing in Switzerland (39.1%) [27].
One of the most common practices of the dental surgeons is prescription of antibiotic post-operatively. The sole excuse is to prevent unforeseen complication. A considerable percentage (72.5%) would cover their patients with antibiotics after a surgical procedure. More specifically, patients undergoing apicectomy procedure for example would be bombarded with antibiotics by nearly 72% of the dental practitioners. In fact as long as the procedure is done in aseptic and at raumatic manner infection of the oral soft tissues is rare. Lindeboom et al. [28], found no differences between clindamycin prophylaxis and placebo in prevention of postoperative infection in endodontic surgical procedures.
Surgical removal of third molars is commonly followed by a course of antibiotics. Salako et al. [14], estimated as high as 89.3% of the respondents would prescribe antibiotics for surgical extraction, in this study however, a fairly lower percentage was found (67.8%). There has been long contentious discussion about the benefits of giving antibiotics in surgical extraction. Recent studies deemed use of antibiotics in post-surgical extraction unnecessary [29,30]. Despite Lodi et al. [31] found no differences between antibiotics and placebo surgical extraction groups in terms of fever, swelling or trismus outcomes, the authors thought a small percentage may be benefited from antibiotics. Nevertheless, antibiotics are advocated for patients undergoing contaminated, longduration surgery [32].
Dry socket or alveolar osteitis is multi factorial with no clear etiology, occurring in fairly low incidence [33,34]. Almost 40% of the respondents would prescribe antibiotics to patients suffering from dry socket. There is no sound evidence as to say dry socket is an infection, therefore, antibiotics are of no value in curing the condition [35].
A quite sensible percentage (57.8%) of dental practitioners would use antibiotics for replantation of avulsed teeth. Systemic use of antibiotics for such conditions has been questioned and the clinical studies do not support such regime, as no value was demonstrated. However, socking the avulsed teeth in antibiotic solution, such as tetracycline has been recommended. Experimental studies however, revealed some positive benefits, the reason behind its current recommendation by the scholars of dental traumatology [36,37].
What is worrying enough is unjustified overuse of antibiotics in conditions related to pulp pathology, which merely need local intervention? In periodontal conditions, except those associated with abscess, the majority of cases require local management. A percentage like 28.3% of the practitioners would use antibiotics in chronic periodontitis cannot be underestimated. On the other hand, consideration of antibiotic therapy in pericoronitis by 76% is justified and acceptable. Nonetheless, from our clinical experience, cases of mild to moderate pericoronitis without signs of spread, frequent flushing with normal saline invariably do well without systemic antibiotics. Necrotizing ulcerative gingivitis is caused by anaerobic microorganisms and warrants specific antibiotic therapy.
Antibiotic therapy for odontogenic sinusitis was considered by 74% of the dental practitioners. Because of the vicinity of upper apices of posterior teeth to the floor of maxillary sinus, there is no doubt of potential infection spread. However, diagnosis of such cases needs to be meticulous to avoid unnecessary overuse of antibiotics.
In spite of the small percentages, antibiotic overuse becomes a real threat when dental practitioners would unwisely use antibiotics for restorative treatment, pulp capping, scaling and polishing, and chronic infection treatment.
The respondents reported similar antibiotics preferences to the previous studies in the region [14,25]. Amoxicillin remained the most commonly used antibiotic in treatment of odontogenic infection of patients not allergic to penicillin (Table 4). The only exception was in case of cellulitis, where Augmentin was preferred by nearly 45% compared to amoxicillin (29.1%). Amoxicillin is semi synthetic drug and stable in presence of gastric acid. It can fight a mixture of facultative and anaerobes. It has good absorption by soft tissues thus advocated in soft tissue infections; it is however poorly absorbed by bone. Augmentin (amoxicillin plus clavulanate) is a broad spectrum antibiotic, which demonstrated high antibacterial effectiveness. It is usually reserved for unresolved infections and immune compromised patient. In case of allergy to penicillin Clindamycin is the drug of choice. Erythromycin had less preference among the practitioners. The odontogenic infections are predominated by Streptococci viridans, Prevotella, Peptostreptococcus, Porphyromonas and Fusobacterium. The recent studies revealed that erythromycin is resistant to the former strains and doubted its benefit in treatment of severe orofacial infection [38]. In fact a previous study considered erythromycin as a historical antimicrobial drug in treatment of odontogenic infections and no more exist on the antibiotics list [39].
Although, metronidazole is the drug of choice in treatment of pericoronitis only 12.7% of the practitioners would use it. Interestingly, Salako et al. [14] reported almost similar percentage (13%). Metronidazole demonstrated the greatest amount of bacterial resistance and is only effective against anaerobes. Therefore, it should be used where anaerobic strains are expected [40].
Cephalosporin and Tetracycline were the least antimicrobial would the practitioners use. There are four generations of cephalosporins with the last generation being more effective against gram-negative bacteria. Cephalosporins however are not a first-line treatment of odontogenic infection [39].
The dental practitioners might go for more than one antibiotic therapy regime, mostly in cases of periapical infection, cellulitis and trismus. Odontogenic infections mostly are mixture of facultative and anaerobic microorganisms; therefore, combination of antibiotics is advocated in certain conditions. Most of the antibiotics used by the dental practitioners are broad spectrum in nature and this enhances the prevalence of antibiotic resistance. On the other hand, Al-Haroni et al. [16] found that Norwegian dentists rely on narrow spectrum antibiotics and their antibiotic prescription is conservative and relatively low compared to physicians.
Antimicrobial drug resistance is a crucial issue for dental professionals. Out of the eight potential contributing factors, widespread use of antibiotics was rated as the most important factor in developing antibiotic resistance followed by inappropriate duration of antibiotic course and use of broad spectrum antibiotics in order. In addition, more than 50% of the respondents thought there are inadequate updates on antibiotic resistance. Considerable percentage of the respondents thought that lack of prescribing guidelines and poor access to culture and sensitivity test are among the major contributory factors in antibiotic resistance development. It is uncommon practice to see culture samples from dental clinics making their way to microbiology labs for culture and sensitivity test. However, a vast recent retrospective study of swabs from odontogenic infections revealed no significant change in microbiological picture or antibiotic sensitivity test over the last 3-4 decades [41]. Therefore, the current antibiotic therapy regimes are good enough to clear the odontogenic infections. In case of severe infections threatening vital structures cultures and antibiotic sensitivity tests should be performed. Approximately half of practitioners would attribute the problem to the lack of prescribing guidelines. In spite of the inconsistency, recently several guidelines have been published [42-45]. The question is that “how often the practitioners are updating themselves with the recent changes?” Yet to be answered! Several practitioners thought that the other factors, such as antibiotic promotion by manufacturer companies and patients’ demand and expectation share a stake in the problem and should not be under estimated.
There is a possibility that the recent graduates might have had updated knowledge on antibiotics use and prescribing guidelines. This was demonstrated by a slightly higher significance in the mean knowledge variable among practitioners who were in practice for less than 5 years (Table 5), reminding that this tests the knowledge but not the clinical skills of the different participated groups.
It should be clear that the current survey looked at the knowledge and attitude of the therapeutic and not the prophylactic aspects of prescribing antibiotics among the practitioners.
In this study sample of dental practitioners practicing in northern emirates of UAE, the practitioners were found to have generally fair knowledge on antibiotics use and abuse. Nevertheless, the study revealed lack of consistency in the rational prescription of antibiotics for many of the dental conditions. Therefore, compliance with the antibiotic guidelines and good practices were breached. Amoxicillin remains on top of the anti microbial drug list commonly prescribed by the dental practitioners in management of odontogenic infections. The knowledge on the potential contributing factors towards development of antibiotic- resistance bacteria among the practitioners was acceptable. Statistically, there were no differences in antibiotic prescription by practitioners’ qualification or gender. However, practitioners with less than 5 years in practice have better knowledge.
Development of antibiotic- resistant bacteria is a global problem and the dental professionals have paramount obligations to reduce the burden. Therefore, academicians need to take serious steps at the institution and community levels. These could be in the form of conducting seminars, continuous dental education courses and sharing lectures in conferences to disseminate updated knowledge on antibiotics use and abuse and enhance the awareness of antibioticresistant bacteria development.
Patients participated in the current study were consented prior participation after detailed explanation of the treatment steps. Approval for the study proposal was obtained from the Ethics and research Committee, RAK medical and Health Sciences University, UAE.
The authors reported no conflicts of interest related to this study.
We would like to declare that we did not receive any funding regarding this research. All the work was done at our own expenses.
Download Provisional PDF Here
Article Type: Research Article
Citation: Alkhabuli J, Kowash M, Shah A (2015) Knowledge and Attitude of Northern Emirates Dental Practitioners towards Antibiotic Prescription and its Resistance. Int J Dent Oral Health 2(3): doi http://dx.doi.org/10.16966/2378-7090.177
Copyright: © 2016 Alkhabuli J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access