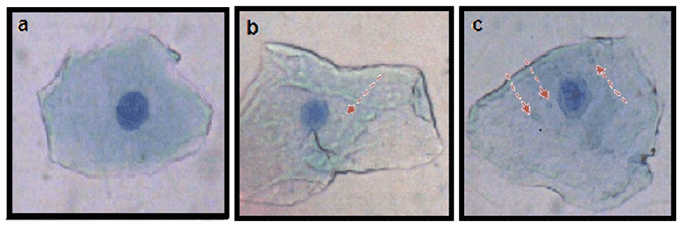
Figure 1: Buccal epithelial cells (a) Control: with no micronucleus (b & c) Cases: with one and three micronuclei respectively.( Giemsa Stain, 40X)

Saif Khan1,* Sadaf Hasan2 Asad U Khan2
1Department of Periodontics and Community Dentistry,Dr Z A Dental College, Aligarh Muslim University, Aligarh, India*Corresponding author: Saif Khan, Department of Periodontics and Community Dentistry, Dr Z A Dental College, Aligarh Muslim University, Aligarh, India, E-mail: saifgood@gmail.com, sadaf11hasan@gmail.com
Objective: To study the genotoxic effect of Chlorhexidine mouthwash on buccal epithelial cells in chronic gingivitis patients.
Methods: Chronic gingivitis patients(n=50) who were exclusively on mechanical plaque measures were taken as Controls(Group A) and chronic gingivitis patients who were on mechanical plaque measures along with adjunct 0.2 % Chlorhexidine mouthwash (n=50) were taken as Cases (Group B). Buccal epithelial cells were taken from these patients and were collected in a buffer and the centrifuged at 8000 rpm for 5 minutes. The cells were fixed with cold methanol (100%). The slides were kept at 37°C overnight and then stained with 5% Giemsa stain. The micronuclei frequency was calculated by observing 2000 nucleated cells per individual slides under microscope.
Results: The average micronuclie frequency in Cases (4.62 ± 0.433) was approximately eight folds greater as compared to the Controls (0.6 ± 0.125). However, the total number of micronucleated cells that were present in Cases and Controls were 140 and 28 respectively. This study was statistically significant with p<0.01.
Conclusion: Our findings with the micronucleus test indicate that the use of anti plaque agent chlorhexidine induces DNA damage resulting in genotoxicity.
Dental plaque; Gingivitis; Chlorhexidine; Mouthwash
Chronic gingivitis and chronic periodontitis are two form of inflammatory disease of tooth supporting structures caused by dental plaque [1]. Mechanical plaque control measures such as tooth brushing and flossing used alone to control dental plaque are inadequate in their efficacy [2]. Chemical plaque control measures such as mouth rinses are used as an adjunct but not as replacement to mechanical plaque control measures [3]. Chlorhexidine mouthwash is considered as the gold standard chemical plaque control measure [4]. The antiplaque action of Chlorhexidine is owing to its strong attraction towards bacterial membranes leading to increased cell permeability at low concentrations and cytolysis and cell death at high concentration [5]. It has been shown that Chlorhexidine has cytotoxicity for gingival cells [6].
Also, Parachloroaniline, a breakdown product of Chlorhexidine has been shown to have mutagenic effects [7]. Micronuclie are biomarkers of genotoxic damge and are formed as a result of chromosomal aberration in the basal cells of the epithelium in which unattached chromosomes or their fragments are excluded from the nucleus [8]. The prevalence of micronuclei in buccal epithelial cells of healthy individuals is rare but it increases on exposure to radiation or some genotoxic agent [9].
The objective of our study was to assess the genotoxic effects of chlorhexidine mouthwash by calculating micronuclei frequency in the buccal epithelial cells of its users.
The study was carried out in patients reporting to the outpatient of Department of Periodontics, Dr Z A Dental College and Hospital, AMU, Aligarh with collaboration of Interdisliplinary Biotechnology unit, AMU, Aligarh.
Chronic gingivitis patients (Probing depth≤3mm, using William Periodontal Probe) who were systemically healthy and consented to be part of our study after explaining them potential risk and benefit were included in the study
The patients enrolled in the study were divided into two groups
Group A or controls: Chronic gingivitis patients (n=50) who were exclusively on mechanical plaque control (Phase I) without any adjunctive chemical plaque control measures.
Group B or cases: Chronic gingivitis patients (n=50) on mechanical plaque control along with adjunct 0.2% Chlorhexidine mouthwash (aqueous base).
The frequency of the Chlorhexidine mouthwash use was twice daily (10ml of 0.2% Chlorhexidine was swished in mouth for 1 minute) and duration of usage ranged from one week to six months in this study.
Plaque index by Sillness and Loe [10] and Gingival index by Loe and Sillness [11] were recorded by William’s Periodontal Probe.
Patients who were smokers, tobacco chewers or with any form of tobacco addiction alcoholics, patients with dental caries or with any dental restoration , orthodontic appliances , removable partial dentures, cast partial dentures were excluded from the study [12-14].
Hundred patients were included this study of which 50 were Controls (Group A) and 50 cases (Group B). The patients age ranged from 13 to 73years in Group A whereas 15 to 70 years in Group B. There were 30 males and 20 females in Group A and 25 males and 25 females in Group B.
The study was approved by Institutional Ethical Committee, of our institute and informed consent was taken from the patients before they were inducted into the study.
Trizma hydrochloride (Tris-HCL), Ethylene diamine tetra acetic acid (EDTA) from SRL, India. Giemsa stain, Sodium Chloride, Methanol and sodium hydroxide pellets from Merk, India.
The buffer was prepared using dissolving 0.1 M EDTA, 0.001M TrisHCL and 0.02 M Nacl in sterile 1litre distilled water and pH of the buffer was maintained at 7.0 using NaOH.
The collection of buccal cells from each participant was done by gently scraping the cheek using a soft bristled toothbrush without causing injury to the buccal mucosa [15]. This toothbrush containing the exfoliated buccal epithelial cells was then swirled into a test tube containing buffer in order to suspend the cells in the buffer solution present in it resulting into a cell suspension [16].
Buccal cells thus collected were washed twice by centrifugation (8000 rpm for 5 minutes) using the buffer solution as given by Surralles et al. [14]. This step of washing inactivated the endogenous DNAases, removed bacterial load and cell debris that would otherwise complicate the scoring [17]. These cells were then smeared on to clean preheated microscopic glass slides and allowed to air dry. The cells were fixed with cold methanol (100%). The slides were kept at 37°C overnight and then stained with 5% Giemsa stain. These slides were observed under microscope to screen 2000 nucleated cells per individual for the presence of micronucleus [18].
All the micronuclie were scored based on criteria given by Tolbert et al. [18]. Micronuclie cells were identified as DNA-containing structures which are separated from the nucleus. The area of micronuclie is about one third of the area of the main nucleus. The micronuclei are usually lighter in staining than the main nucleus (Figure1). The cells with intact cytoplasm without any overlap with adjacent cells and free of debris were taken for counting of micronuclei [18].
Statistical analysis was carried out by Student’s unpaired‘t’ test to compare micronuclie frequency in Cases (Group B) and Controls (GroupA).
The frequency of the Micronuclei (MN), Gingival index (GI) and Plaque index (PI) values of Group A and Group B of this study are shown in Table 1 and 2 respectively, whereas Table 3 shows the frequencies of micro nucleated cells in cases (Group B) and controls (Group A). It is clearly depicted from the Table 3 that the average MN frequency in Group B (4.62 ± 0.433) is approximately 8 folds greater as compared to the Group A (0.6 ± 0.125). However, the total number of micro nucleated cells that are present in Group A and Group B 28 and 140, respectively (Table 1 and Table 2). This study was statistically significant with p<0.01.

Figure 1: Buccal epithelial cells (a) Control: with no micronucleus (b & c) Cases: with one and three micronuclei respectively.( Giemsa Stain, 40X)
The aim of this study was to assess the genotoxic potential of Chlorhexidine mouthwash by counting the number of micronuclei in the exfoliated buccal epithelial cells of its users to compare with that of the controls. Micronucleus assay in exfoliated buccal epithelial cells has been regularly used in genetic biomonitoring of populations exposed to several genotoxic chemicals, such as tobacco, pesticides, and alcohol and its increased frequency shows risk for cancer [19,20].
The main advantage of the micronucleus assay is that it is relatively easy to of score, cost-effective, requires limited resources and larger numbers of cells can be scored with precision and also it reflects genomic instability [21].
In our study we calculated the micronuclei frequency in cases and controls in the exfoliated buccal epithelial cells. As shown in the Table 1, the total number of micronuclei observed in Group A was 30 where as in Group B was 231 (Table 2). The mean frequency of micronuclei observed in Cases (Group B) and Controls (Group A) were 4.62 ± 0.433 and 0.6 ± 0.125 respectively (Table 3). These observations show that micronucleus frequency in buccal epithelial cells of patients with mechanical plaque control along with adjunctive Chlorhexidine (Group B) was statistically significant than in exfoliated buccal epithelial cells in persons who were exclusively on mechanical plaque control without any adjunctive chemical plaque control measure (Group A). These findings are consistent with that of Carlin V et al. who observed 1.8% increase in micronucleus frequency in buccal epithelial cells as compared to that before exposure (0.27%) in patients who were given adjunct 15 ml of 0.12% Cholrhexidine mouth rinse twice daily for two weeks. The study was statistically significant (p<0.05) [22].
Also, Erdemir et al., in their study compared micronucleus frequency in 28 patient after exposure to three commercially available mouth rinses viz ; Klorhex (0.2%Chlorhexidine gluconate), Andorex (0.15% Benzydamine HCL and 0.12% Chlorhexidine Gluconate), Tanflex (0.15% Benzydamine HCL) twice daily for one week on buccal epithelial cells by Micronuclie test. Physiologic saline was used in control group. Micronuclie frequency was significantly increased after 1 week of treatment of the mouthwashes (P<.05) [12].
Moreover, Eren K et al. in their study had 13 volunteers who rinse their mouths with 0.12% Chlorhexidine solution for 18 days. Buccal epithelial cells and peripheral blood lymphocytes were obtained from these volunteers at baseline and the end of the experimental period. Alkaline comet assay was used to analyse 100 cells per subject for the DNA damage. A statistical significant increase was seen in the damaged buccal and blood cells after the Chlorhexidine application (P<0.001).The mean grade of damage in buccal cells was statistically different from that in blood cells (P<0.001) [23].
In wistar rats, Ribeiro et al. investigated genotoxicity of 0.12% chlorhexidine digluconate on peripheral blood and oral mucosal cells by the single cell gel (comet) assay and micronucleus test and found statistically significant increase of DNA damage in leukocytes and oral mucosal cells of the chlorhexidine digluconate treated group [24].
These above studies provide the evidence about the genotoxicity of Chlorhexidine on buccal epithelial cells which are consistent with our findings. Also, Chlorhexidine at very miniscule concentration of 0.004% resulted in impaired cellular function and or cell death of fibroblasts [25].
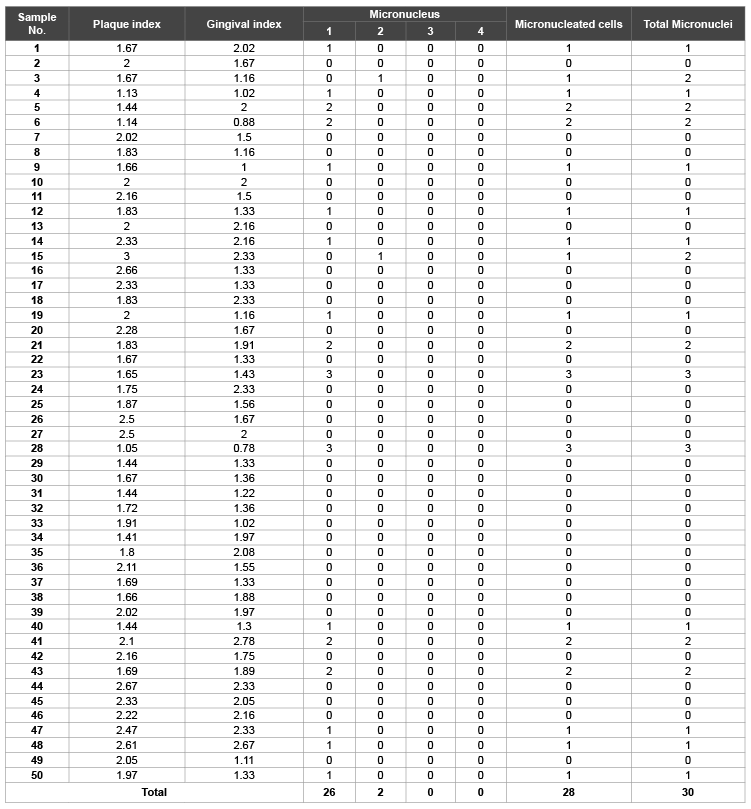
Table 1: Micronuclie (MN) distribution in controls (Group A)
Moreover, exposure of fibroblasts to solutions of 0.12% Chlorhexidine for as little as 30 seconds produced the same results [26]. Thus Chlorhexidine has toxic effects on a variety of eukaryotic cells and cytotoxic mechanism is presumed to be related to electrostatic interaction [27].
It has been shown that about 30% of a10 ml one minute rinse of 0.2% Chlorhexidine is bound in the oral cavity and is subsequently released over next 8-12 hours and weak concentrations were detected in saliva even after 24 hours of rinsing [28].
Moreover, chlorhexidine concentrations much below than used in clinical dentistry have been seen to cause cell injury, cell death and inhibition of protein synthesis in human fibroblast culture and HeLa cell cultures [25].
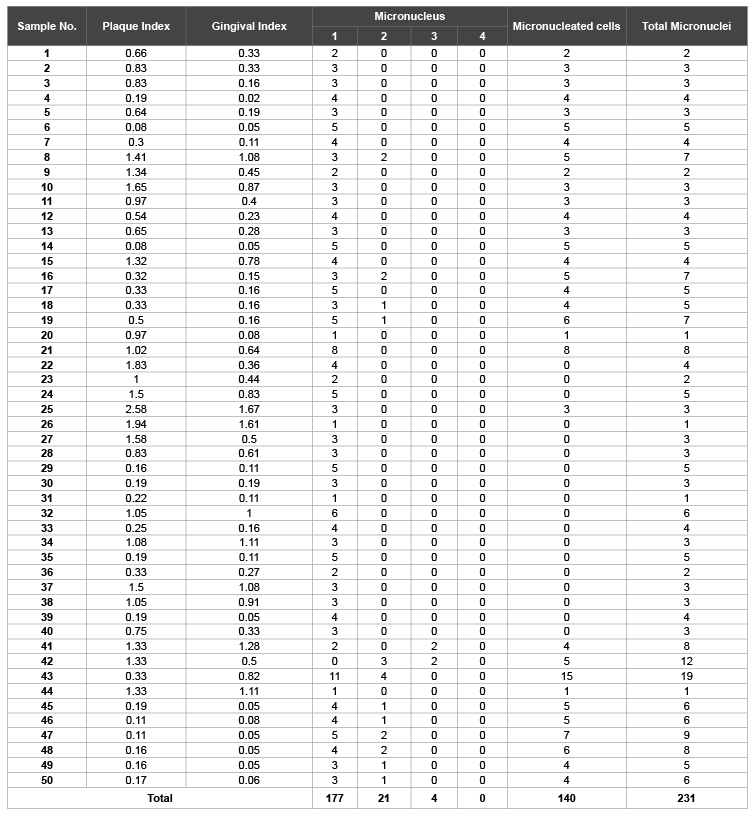
Table 2: Micronuclie (MN) distribution in cases (Group B)
In this study chronic gingivitis patients on mechanical plaque control with adjunct 0.2% Chlorhexidine mouthwash had statistically increased micronuclei frequency than patient exclusively on mechanical plaque control. Therefore, as micronuclie is an established marker of genotoxicity, our results apparently show that the use of chlorhexidine results in high rate of DNA damage leading to genotoxicity.
In conclusion, our findings with the micronucleus test indicate that the use of anti plaque agent chlorhexidine induces DNA damage in buccal epithelial cells resulting in genotoxicity and therefore it should be used judiciously in clinical practice.
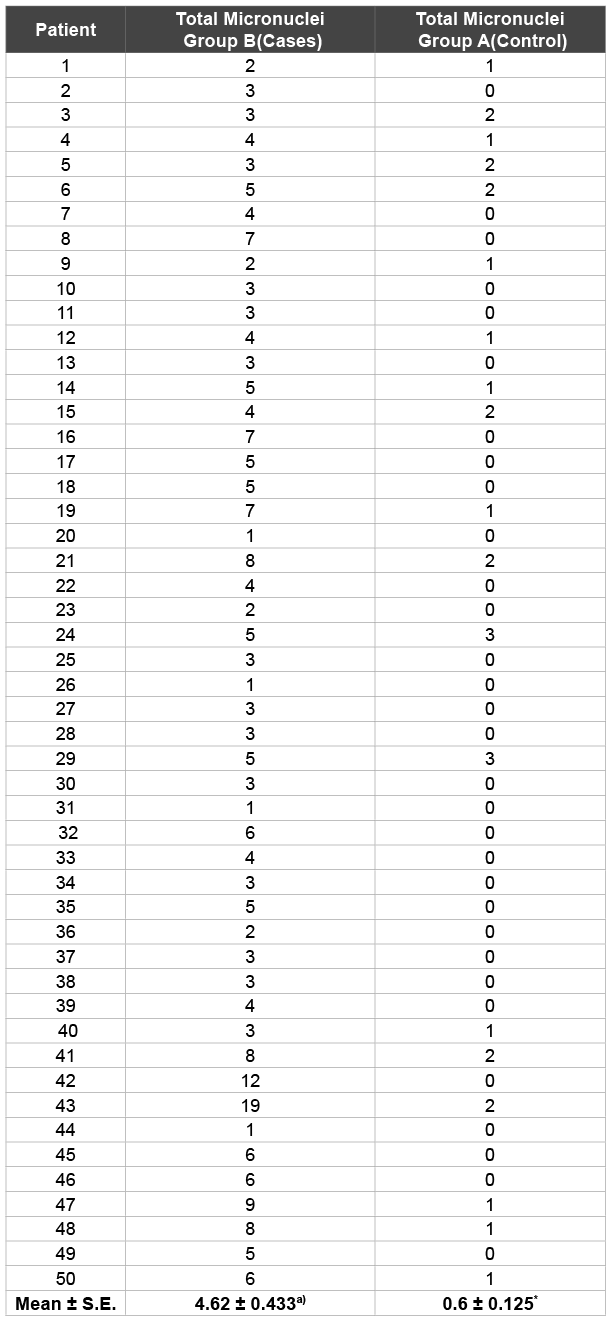
Table 3: Micronuclei frequency per 2000 buccal cells/ individual of 50
cases and 50 controls.
P* <0.01 (statistically significant with respect to control)
None
The authors are thankful to their institutions for providing all the facilities for the study.
Download Provisional PDF Here
Article Type: Research Article
Citation: Khan S, Hasan S, Khan AU (2015) Genotoxic Effects of Chlorhexidine Mouthwash on Buccal Epithelial Cells. Int J Dent Oral Health 2(2): doi http://dx.doi.org/10.16966/2378-7090.146
Copyright: © 2015 Khan S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access