
Full Text
Peripheral Odontogenic Fibroma in a Young Child: A Literature Review and a Report of a Rare Case
Talia Becker1*, Ori Platner21Department of Oral Pathology and Oral Medicine, The Maurice & Gabriela Goldschleger School of
Dental Medicine, Tel Aviv University, Israel
2*Department of Periodontology and Dental Implantology, The Maurice & Gabriela Goldschleger School of Dental Medicine, Tel Aviv University, Israel
*Corresponding author: Dr. Talia Becker, Department of Oral Pathology and Oral Medicine, The Maurice & Gabriela Goldschleger School of Dental Medicine, Tel Aviv University, Tel Aviv, Israel, Tel: +972-50- 5808886; E-mail: becktalia@gmail.com
Abstract
Peripheral odontogenic tumors (POTs) are uncommon, and even rarer in the pediatric population. A peripheral odontogenic fibroma (PODF) is the most numerous POT in the general and pediatric populations. The purpose of this paper was to report an unusual case of PODF in a very young child who was born at 29 weeks’ gestation. The literature on PODFs in the pediatric population is reviewed.
Keywords
Peripheral odontogenic fibroma; Pediatric; Peripheral odontogenic tumor
Introduction
Peripheral odontogenic tumors (POTs) include the extraosseous counterparts of ameloblastomas, calcifying epithelial odontogenic tumors (CEOTs), squamous odontogenic tumors, calcifying cystic odontogenic tumors (CCOTs), adenomatoid odontogenic tumors (AOTs), ameloblastic fibromas, odontomas, odontogenic fibromas, and odontogenic myxomas, some of which are exceedingly rare. A comprehensive study carried out in an oral pathology biopsy service demonstrated that POTs represented only 0.05% of all submitted biopsy specimens [1]. Although POTs are uncommon among children, peripheral variants of odontogenic fibromas (e.g., CCOTs, AOTs, ameloblastic fibromas and odontomas) have been documented in the pediatric population. Peripheral odontogenic fibromas (PODFs) were found to be the most common of all POTs in general [1] and the most common POT in the pediatric population [2]. We report a case of PODF in a 3.9-year-old child whose only remarkable feature in her past history was being born at 29 weeks’ gestation. The literature on PODFs in the pediatric population is reviewed.
Case Report
A 3.9 -year-old girl was referred by the community dentist to the Department of Oral Medicine in a University health center. The mother had noted an “odd-looking tooth” located at the anterior mandible. The child’s past history was remarkable for premature induced birth (29th week of gestation) due to maternal uterine hematoma. The newborn was intubated and ventilated immediately after birth and remained so for a few days. She was also diagnosed as having Klebsiella pneumoniae-related sepsis for which she received antibiotic treatment. Other than infantile hemangiomas on the skin of her lower back, the child otherwise appeared to be healthy and fully developed, both cognitively and physically.
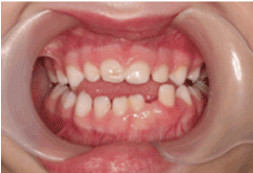
Clinical examination revealed the patient’s face to be symmetrical with a normal skin tone. There were neither palpable submandibular or cervical lymph nodes nor any temporomandibular joint abnormalities. An anterior open bite was explainable by habitual sucking of pacifiers. The midline of the lower dental arch was approximately 1 mm to the left compared to the midline of the upper dental arch (Figure 1). The child was caries free, and her oral hygiene was highly satisfactory. The right maxillary central and lateral incisors demonstrated enamel hypoplasia. The left mandibular deciduous lateral tooth was approximately 3 mm wider compared to its right-sided counterpart, suggesting single tooth macrodontia. This tooth was also over-erupted, with obvious distal displacement and grade I mobility (Figure 2). The attached buccal and lingual gingivae surrounding it were symmetrically hyperplastic (Figure 2), with a smooth surface lining of normal-colored oral mucosa and firm consistency on palpation. There were no bloody or purulent discharges upon probing, and her periodontal status was otherwise sound. Occlusal trauma on the tooth was ruled out since it did not have occlusal contacts either in the resting position or in working/ nonworking relations. Periapical radiographs revealed that the lower left lateral incisor had an open apex, while its right-sided counterpart had a closed apex (Figure 3). In addition, the pulp chamber was disproportionately wide relative to tooth dimensions. There was obvious distal tooth displacement (Figure 3).

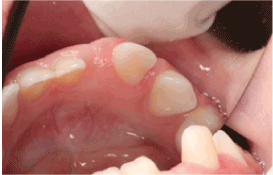
Figure 2:The attached buccal and lingual gingivae surrounding the tooth appear hyperplastic and covered by normal-looking oral mucosa.
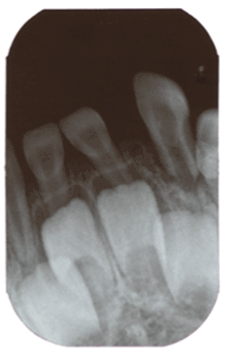
Figure 3:Periapical X-ray. The lower left lateral presenting an open apex, a wide pulp chamber and tooth displacement.
The clinical differential diagnosis included localized reactive hyperplastic lesions (LRHLs), such as peripheral ossifying fibromas (POFs), non- odontogenic benign tumors, such as myofibromas, and POTs, such as PODFs.
After the child was sedated with midazolam (7 mg) and nitrous oxide, the lower left lateral incisor was extracted and the hyperplastic gingiva was excised by scalpel and submitted to microscopic examination. The hematoxylin and eosin-stained slides revealed islands of inert odontogenic epithelium in a fibrous stroma. The remaining connective tissue contained mild chronic inflammatory infiltrate (Figure 4). The microscopic findings were consistent with the diagnosis of a PODF.
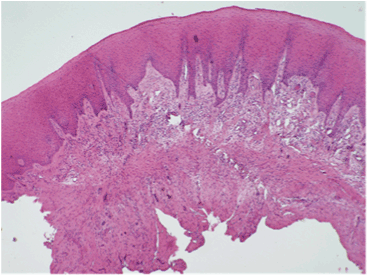
Figure 4:Excisional biopsy showing fibrous stroma lined by keratinizing stratified squamous epithelium with alternating areas of hypo- and hypercellularity. Small islands/rosettes of epithelium can be discerned, consistent with inert odontogenic epithelium. The rest of the connective tissue contained mild chronic inflammatory infiltrate. Peripheral odontogenic fibroma was diagnosed (Hematoxylin and eosin stain, original magnification x 40).
There was no evidence of recurrence, either clinically (Figure 5) or radiologically (Figure 6) at the one-week, 3-month, 8-month and 1-year follow-up visits. The extraction area demonstrated normal healing. There were no signs of inflammation, and no symptoms were reported. The radiographic periapical image taken at eight months following the procedure demonstrated normal appearance of the bone and of the deciduous teeth and adjacent tooth germs near the extraction area. Since recurrences have been reported in the literature [2],the child is scheduled for follow-up appointments every four months.
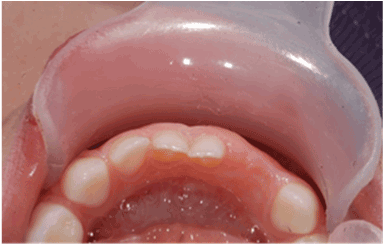
Figure 5: Follow-up at one year following the excisional biopsy revealing satisfying tissue recovery and no recurrence of the lesion (occlusal view).
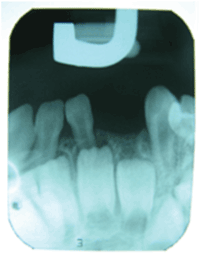
Figure 6: Periapical X-ray at one year following the excisional biopsy demonstrating normal appearance of bone in the extraction area of the lower left lateral.
Discussion
PODF is the only POT that is more frequent than its central counterpart. The mean age at diagnosis for the population at large is 32.3 years, and there is a slight female preponderance. The mandibular incisor/canine and premolar areas are the most prevalent sites, affecting both the buccal and lingual aspects of the gingival [1]. PODFs present as exophytic gingival masses that histopathologically show unencapsulated fibrous connective tissue with inert odontogenic epithelium [3]. Table 1 lists a summary of age, gender and location of POFs in the pediatric populations reported in the literature.
We defined “pediatric population” in accordance with Buchner et al. [1] as being 0- to 19-year-olds. We reviewed the clinical information of all the documented cases of PODFs and POTs among pediatric patients that appeared in the English-language literature. Medline’s PubMed interface was searched using the keywords “peripheral” combined with “odontogenic tumor”, “odontogenic fibroma”, “ameloblastoma”, “calcifying epithelial odontogenic tumor”, “squamous odontogenic tumor”, “calcifying cystic odontogenic tumor”, “adenomatoid odontogenic tumor”, “ameloblastic fibroma”, “odontoma”, “odontogenic fibroma”, and “odontogenic myxoma”.
| Case No | Study | Age (years) | Pediatric cases | Gender | Location |
|---|---|---|---|---|---|
| 1 | Buchner et al.[1] | 2 | 1 | M | Maxilla |
| 2 | 18 | 1 | F | Maxilla | |
| 3 | Weber et al. [7] | 8 | 1 | F | Diffused |
| 4 | 3 | 1 | M | Diffused | |
| 5 | Daley et al. [4] | 19 | 1 | M | Mandible |
| 6 | 13 | 1 | M | Mandible | |
| 7 | 18 | 1 | F | Mandible | |
| 8 | 12 | 1 | F | Maxilla | |
| 9 | 14 | 1 | M | Maxilla | |
| 10 | 16 | 1 | F | Unknown | |
| 11 | Siar & Ng [6]* | 05-Dec | 1 | Unknown | Unknown |
| 12 | 4 | 1 | Unknown | Unknown | |
| 13 | 9 | 1 | Unknown | Unknown | |
| 14 | Martelli-Júnior et al. [5] | 11 | 1 | Unknown | Unknown |
| 15 | Buchner et al. [1] | 03-Dec | 1 | F | Maxilla |
| 16 | 18 | 1 | M | Mandible | |
| 17 | 16 | 1 | F | Mandible | |
| 18 | 12 | 1 | F | Maxilla | |
| 19 | 15 | 1 | M | Mandible | |
| 20 | 12 | 1 | F | Maxilla | |
| 21 | Alaeddini et al. [8] | 17 | 1 | M | Mandible |
| 22 | 18 | 1 | M | Mandible | |
| 23 | 12 | 1 | M | Mandible | |
| 24 | 8 | 1 | M | Mandible | |
| 25 | Ritwik& Brannon [2] **Current case | 9 | 1 | M | Mandible |
| 26 | Unknown | 35 | Unknown | Unknown | |
| 27 | 4 | 1 | F | Mandible |
Table 1: Systemic diseases chosen for the study along with the most frequent forms of the disorders seen in the sample.
One review of the English language literature specific to PODFs in children aged 0-19 years [2] revealed that 35 of cases of all ages (n=151, 23.2%) involved children, the youngest of which was five years old. Another case series described 6 of all 23 patients (26%) being between 12-18 years of age [1]. Daley and Wysocki’s survey [4] revealed that 6 of 36 new cases of all ages were within 0-19 years of age, the youngest being 12 years old. Reports on pediatric populations under the age of 9 years are uncommon, although a few cases have been documented, including two that involved infants [5,6] and one each of a 2-year-old [3], a 3-year-old [7], a 4-year-old [6], and a 5-year-old [2], and two others, each describing an 8-year-old [7,8]. We believe our case to be the fifth to document a PODF in a child younger than four years of age.
Overall, POTs comprise only 0.05% of all submitted oral biopsy specimens, with a relative frequency of 51% among the various histopathologic types of PODFs, 29% of peripheral ameloblastomas and 13% of peripheral calcifying cystic odontogenic tumors. Other histopathological types of POT are sporadic [1]. POTs are uncommon among children patients. One pediatric case of PCCOT was documented in a series of 6 cases (16.6%), [1] and another was reported in a single case report [9]. The age range of peripheral adenomatoid odontogenic tumors in 15 out of 15 pediatric cases was 3 to 19 years (mean, 11.9 years) [10-13]. The age range of peripheral ameloblastic fibromas in 7 out of 7 pediatric cases was 1 to 8 years (mean, 4 years) [1,10,14-16]. The age range of peripheral developing odontoma in 8 out of 8 pediatric cases was 1 to 14 years (mean, 6.6 years) [10]. There is no documentation of the peripheral counterparts of ameloblastomas in pediatric cases [1], keratocystic odontogenic tumors [10], squamous odontogenic tumors [17] or odontogenic myxomas [18].
Like other tissues of the body, the oral structures can be affected by premature birth. The effects of prematurity in the mouth are associated with enamel hypoplasia, crown dilacerations, palatal distortions and distortions of dental arches [19-30]. There is no published correlation between preterm birth and neoplastic lesions, with the exception of infantile hemangiomas [31].
This case report and literature review highlights the rarity of PODFs among young children. Any associations between odontogenic lesions and premature birth are still unclear.
References
- Buchner A, Merrell PW, Carpenter WM (2006) Relative frequency of peripheral odontogenic tumors: a study of 45 new cases and comparison with studies from the literature. J Oral Pathol Med 35:385-391.[Ref.]
- Ritwik P, Brannon RB (2010) Peripheral odontogenic fibroma: a clinicopathologic study of 151 cases and review of the literature with special emphasis on recurrence. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 110:357-363.[Ref.]
- Buchner A, Ficarra G, Hansen LS (1987) Peripheral odontogenic fibroma. Oral Surg Oral Med Oral Pathol 64:432-438.[Ref.]
- Daley TD, Wysocki GP (1994) Peripheral odontogenic fibroma. Oral Surg Oral Med Oral Pathol 78:329-336.[Ref.]
- Martelli-Júnior H, Mesquita RA, de Paula AM, Pêgo SP, Souza LN (2006) Peripheral odontogenic fibroma (WHO type) of the newborn: a case report. Int J Paediatr Dent 16:376-379.[Ref.]
- Siar CH, Ng KH (2000) Clinicopathological study of peripheral odontogenic fibromas (WHO-type) in Malaysians (1967-95). Br J Oral Maxillofac Surg 38:19-22.[Ref.]
- Weber A, van Heerden WF, Ligthelm AJ, Raubenheimer EJ (1992) Diffuse peripheral odontogenic fibroma: report of 3 cases. J Oral Pathol Med 21:82-84.[Ref.]
- Alaeddini M, Salehizadeh S, Baghaii F, Etemad-Moghadam S (2010) A retrospective analysis of peripheral odontogenic fibroma in an Iranian population. J Oral Maxillofac Surg 68:2099-2103.[Ref.]
- Resende RG, Brito JA, Souza LN, Gomez RS, Mesquita RA (2011) Peripheral calcifying odontogenic cyst: a case report and review of the literature. Head Neck Pathol 5:76-80.[Ref.]
- Ide F, Mishima K, Saito I, Kusama K (2008) Rare peripheral odontogenic tumors: report of 5 cases and comprehensive review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 106:e22-28.[Ref.]
- Philipsen HP1, Reichart PA, Siar CH, Ng KH, Lau SH, et al. (2007) An updated clinical and epidemiological profile of the adenomatoid odontogenic tumour: a collaborative retrospective study. J Oral Pathol Med 369:383-393.[Ref.]
- Takeuchi Y, Takai Y, Itoh A, Nakayama K, Horii M, et al. (1988) Two cases of adenomatoid odontogenic tumor difficult to diagnose clinically. Aichi-Gakuin J Dent Sci 26:401-406.[Ref.]
- Jham BC, Passos JB, do Carmo MAV, de Oliveira Gomes C, Mesquita RA, et al. (2006) Adenomatoid odontogenic tumor originated in the periodontal ligament. Oral Oncol Extra 42:268-271.[Ref.]
- Nara N, Takenoshita K, Ishibashi H (2003) A case of peripheral amelo-bastic fibroma. Pediatr Oral Maxillofac Surg 13:35.
- Darling MR, Daley TD (2006) Peripheral ameloblastic fibroma. J Oral Pathol Med 35:190-192.[Ref.]
- Abughazaleh K, Andrus KM, Katsnelson A, White DK (2008) Peripheral ameloblastic fibroma of the maxilla: report of a case and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 105:e46-48.[Ref.]
- Baden E, Doyle J, Mesa M, Fabié M, Lederman D, et al. (1993) Squamous odontogenic tumor. Report of three cases including the first extraosseous case. Oral Surg Oral Med Oral Pathol 75:733-738.[Ref.]
- Aytac-Yazicioglu D, Eren H, Görgün S (2008) Peripheral odontogenic myxoma located on the maxillary gingiva: report of a case and review of the literature. Oral Maxillofac Surg 12:167-171.[Ref.]
- Seow WK (1997) Effects of preterm birth on oral growth and development. Aust Dent J 42:85-91.[Ref.]
- Seow WK (1981) Enamel hypoplasia in the primary dentition: a review. ASDC J Dent Child 58:441-452.[Ref.]
- Seow WK, Brown JP, Tudehope DI, O’Callaghan M (1984) Defects in the primary dentition of children born prematurely with very low birth weight and neonatal rickets. Pediatr Dent 6:88-92.
- Seow WK, Humphrys C, Tudehope DI (1987) Increased prevalence of developmental dental defects in low-birth-weight children: A controlled study. Pediatr Dent 9:221-225.[Ref.]
- Seow WK, Masel JP, Weir C, Tudehope DI (1989) Mineral deficiency in the pathogenesis of enamel hypoplasia in prematurely-born, very low birthweight children. Pediatr Dent 11:297-302.[Ref.]
- Seow WK, Brown JP, Tudehope DI, O’Callaghan M (1984) Developmental defects in the primary dentition of very low birth weight infants: Adverse effects of laryngoscopy and prolonged endotracheal intubation. Pediatr Dent 6:28-31.[Ref.]
- Seow WK, Brown JP, Tudehope DI, O’Callaghan M (1985) Effect of neonatal laryngoscopy and endotracheal intubation on palatal symmetry in two to five-year-old children. Pediatr Dent 7:30-36.[Ref.]
- Seow WK, Humphrys C, Mahanonda R, Tudehope DI (1988) Dental eruption in low birth-weight, prematurely-born children: a controlled study. Pediatr Dent10:39-42.[Ref.]
- Seow WK (1996) A study of the development of the permanent dentition in very-low birthweight children. Pediatr Dent18:379-384.[Ref.]
- Lai PY, Seow WK, Tudehope DI, Rogers Y (1997) Enamel hypoplasia and dental caries in very-low birthweight children: A case-controlled, longitudinal study. Pediatr Dent 19:42-49.[Ref.]
- Seow WK, Perham S (1990) Enamel hypoplasia in prematurely-born children: A scanning electron microscopic study. J Pedod 14:235-239.[Ref.]
- Seow WK, Perham S, Young WG, Daley T (1990) Dilaceration of a primary maxillary incisor associated with neonatal laryngoscopy. Pediatr Dent 12:321-324.[Ref.]
- Garzon MC, Drolet BA, Baselga E, Chamlin SL, Haggstrom AN, et al. (2008) Comparison of infantile hemangiomas in preterm and term infants: a prospective study. Arch Dermatol144:1231-1232.[Ref.]
Download Provisional PDF here
Article Information
Article Type: Case Report
Citation: Backer T, Platner O (2015) Peripheral Odontogenic Fibroma in a Young Child: A Literature Review and a Report of a Rare Case.J Dent Oral Health, Volume1.1: http://dx.doi.org/10.16966/2378-7090.102
Copyright: © 2015 Backer T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
SCI FORSCHEN JOURNALS
All Sci Forschen Journals are Open Access
