Introduction
The scope of surgical management of deformities in the cranio-maxillofacial region, whether they are congenital, developmental or acquired, is to reestablish facial symmetry and restore form and function [1,2]. Diagnostic imaging plays an important adjunct to achieve these goals. Advances in imaging techniques have greatly improved the treatment outcome and enhanced the success rate of surgical procedures. However, few problems are still faced by surgeons, notably accurate repositioning of skeletal structures in optimal three dimensional planes, restoration of ideal orbital volume, compromised visualization of deeper structures, removal of foreign bodies and assessment of variations in abnormal growth patterns. This owes to the dependence of two dimensional imaging to treat a three dimensional (3D) problem. The computer-assisted navigational surgery was introduced in maxillofacial surgical practice [3-6]. It was first applied by Watanabe in 1987 in neurosurgical procedures [7]. It allows direct access to specific targeted areas through smaller incisions, thus making surgery less invasive, reliable and reducing overall operation time. Surgical navigation hypothetically resembles the global positioning system used in automobiles and has three components: a localizer, which is similar to the satellite in space; a surgical probe, which represents the track waves emitted by the global positioning system (GPS) in the vehicle and a computerized tomography (CT) scan data set, which is analogous to a road map (Figure 1). Apart from CT scan, the data set can also use magnetic resonance imaging and positron emission tomography [8]. There are several types of surgical navigation systems available today.
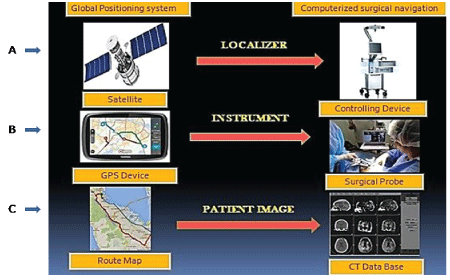
Figure 1: Photograph showing the computerized surgical navigation and its resemblance of each of its basic components with global positioning system.
(A) The localizer (main controlling device for integration, replication and virtual reproduction of anatomical structures) works as a satellite.
(B) A Surgical probe which sends signals through infrared diodes or pointers and mimics the GPS system in the vehicle, as it emits track waves.
(C) A Computerized tomography database, for identification of abnormalities and confirming postoperative surgical results, is similar to the road map depicted in GPS system for route navigation.
Optical tracking system
In this system, localized points in the surgical field can be viewed in three dimensional positions, through three or two linear cameras. The points are either infrared emitting diodes or passive high reflecting spheres. This system provides high accuracy, however only drawback is that the line of sight between the cameras and tracked points should not be obstructed [9,10].
Screen-based systems
Columbia scientific SIM/Plant software (Columbia scientific, USA): This software helps the surgeon in visualization of implants being inserted surgically through CT scan images. It also provides a 3 dimensional picture of maxillofacial regions with accurate locations of vital structures and helps in the measurement of quality of bone [11,12].
VISIT surgical navigation software (Vienna, Austria): This software aids in simulation of dental implant positions during treatment planning phase. Intraoperatively the drill is visualized by oblique reformatting beside the drill part and the images are changed according to the tracking system. VISIT is popularly used in placement of dental and extraoral implants in maxillofacial region [13,14].
Vector vision (Brainlab, Munich, Germany): This system uses passive marker technology, hence any surgical instrument can be immediately integrated in the operation virtually. The position of the instrument is located and controlled relatively to their positions on preoperative images. This system allows image guided microscopy functional mapping, automatic image fusion, which helps in guiding osteotomies and removal of foreign bodies [15,16].
Stryker leibinger navigation system (Stryker, Leibinger, Freiburg, Germany): In this system a two-way communication takes place between the wireless instrument and navigation system. The surgeon can control patients image data through the handpiece, therefore eliminating the need of touch screen controls [17,18].
Optical tracking system microscope (OTS, Radionics, Tyco Healthcare Group, USA): This optical tracking system precisely matches the preoperative images with the patient’s anatomy and enables the surgeon to track various instrument, during surgery. The location of the instruments and their trajectories are displayed on the screen in either two or three dimensional views [6,19].
Projected image-based systems
Surgical microscope navigation system (SMN, Zeiss, Oberkochen, Germany)
This system is a medical version of navigational target locator used by fighter pilots to fire missiles by aiming at a computer plotted image. It uses a computer driven navigational microscope to provide intraoperative three dimensional view even before the incision is placed [20,21]. It transfers scans of surgical area to the computer, which in turn projects the information onto the lens of the navigational microscope positioned above the patient. It allows the surgeon to follow a planned surgical path, similar to a road map.
Surgical segment navigator (SSN, Carl Ziess, Germany)
It is based on infrared technology and the preoperative data is transferred to the surgical site through infrared transmitters tracked by an infrared camera. The system comprises of an infrared positioning device two reference frames, an infrared pointer and an infrared camera (Figure 2). It can be used in repositioning of fractured segments, osteotomy cuts, positioning of artificial joints and implants. All data are displayed numerically and graphically on the monitor [22,23].
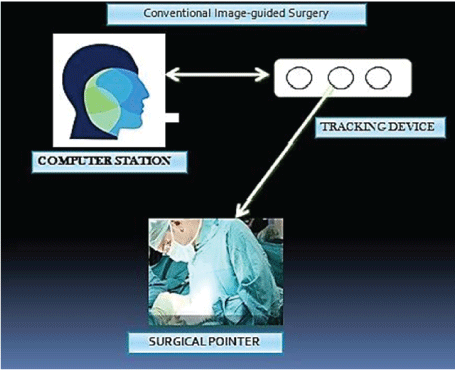
Figure 2: Photograph showing the principle of conventional image guided surgery. A Surgeon uses a pointer, while performing the surgery to correlate between pre-operative images and surgical field. Margin of error is more and is difficult for interpretation and surgical planning.
Microscope-assisted guided interventions (MAGI)
It basically works by projecting the images into the binocular optics of a calibrated and tracked surgical microscope (Figure 3), with an accuracy of 1mm [24]. It works on the principle of stereo augmented reality system, developed by Kings College London [25]. The system consists of a Leica binocular operating microscope adapted by incorporating two purposebuilt monochrome displays and an Optotrak localizer (Northen Digital Inc.). Changes in the position of the microscope are tracked using an array of 13 infrared emitting diodes mounted on a bracket rigidly attached to the microscope assembly. Patient’s position is established by tracking 6 such diodes fixed to a custom-built locking acrylic dental splint.
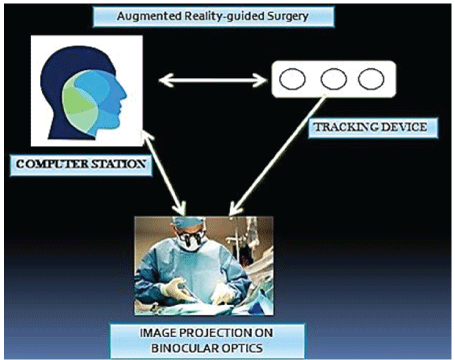
Figure 3: Photograph showing the principle of augmented reality guided surgery. The image is directly projected on to the binocular optics of the surgeon, while performing surgery, over the operating field. Any pathologies, foreign bodies are easily identified also reconstruction on three dimensional planes becomes feasible.
Electromagnetic tracking systems
This system is entirely wireless, thus does not have any lineof-sight restrictions like other systems. But metal instruments can disrupt the electromagnetic waves and hamper the accuracy of the procedure [26,27].
Heat mounted display system (ARTMA)
Through this system, the surgeon can see virtually images of anatomical structures directly on display of headset or endoscope. It is a type of augmented reality system [28,29].
Instatrak (GE medical systems)
This system helps the surgeon to mark critical zones preoperatively, which can be seen during the surgery. Through the development of the magnetic intelligence algorithm from the manufacturer, the system automatically detects and compensates for metal in the field resulting in improved clinical accuracy and reliability [30,31].
Mechanical tracking systems
Viewing wand (ISG, Mississauga, Ontario, Canada)
It was the first frameless neuronavigation device used and consists of an articulated mechanical arm (FARO Technologies) with a hand-held probe, used by the surgeon as pointer. The mechanical operating arm carries 6 joints and has 6 degrees of freedom working as a digitizer, and is interfaced to a computer graphics workstation [21,32].
Use of surgical navigation in oral and maxillofacial surgery
Surgical navigation has great potential in various specialities and provides precision based surgical procedures in areas of complex anatomy, as in maxillofacial region. Recent advances in this field have offered new prospects for orthognathic surgery, reconstruction of facial bones, implantology and Temporo Mandibular Joint (TMJ) disorders.
Orbital reconstruction
High velocity impacts, mostly results in shattered orbits, with increase in volume, herniation of periorbital contents into the sinus and cranial neuropathies. Despite the use of advanced biocompatible materials by experienced surgeons [33], it is still difficult to replace and recontour anatomical landmarks with precision and predictability [4,34-36]. Use of intraoperative navigation with proper preoperative planning have ensured accurate positioning of plates in less visualized anatomic regions [37] described a semiautomatic procedure to use titanium meshes mirrored and constructed as the unaffected side through CT scans and placed using frameless stereotaxy with three infrared cameras, controlling the pointer by integrated light emitting diodes.
Maxillofacial reconstruction
Defects causing discrepancies in mandibular continuity or palatal integrity, due to tumor resection or trauma, results in psychological distress and severe debilitating conditions for the patient. Free fibular osteocutaneous flap has been used by surgeons throughout the world, since it was introduced by Hidalgo [38,39] to reconstruct maxillo-mandibular defects and has proven to be a highly successful and reliable option. Despite its wide acceptance, two school of thoughts lures upon its method of fixation.
Tumor resection
Intraoperative navigation has been proposed as a means to delineate resection margins during extirpative tumor surgery in the craniomaxillofacial skeleton [40-42]. Several reports have highlighted the value of this technology in improving the precision in which tumors are resected, while minimizing the amount of uninvolved tissues. In addition, surgery involving the skull base, pterygomaxillaryfossa or infratemporal fossa, including temporomandibular joint (TMJ) ankylosisrelease [3] may be performed with an added degree of safety with respect to surrounding vital structures (Figure 4). Finally, osteotomies may be accurately positioned based on a presurgical image so that preformed implants, bone grafts or free flaps may be inset into the defect in an effort to increase operative efficiency and accuracy.
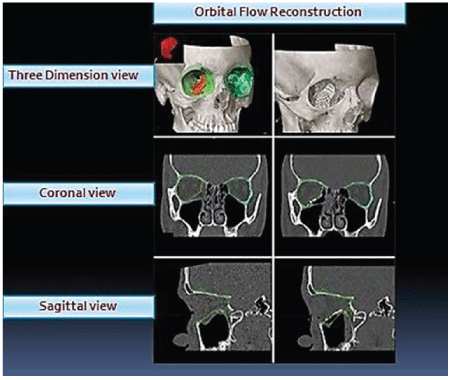
Figure 4: Photograph showing intra operative navigation system for accurate assessment of orbital volume in case of trauma with comparison from normal side and its planning in proper anatomical reconstruction.
Orthognathic surgery
Preoperative computer imaging and intraoperative navigation are useful for planning complex surgical movements of the craniofacial skeleton. Using recently designed Computeraided design/ Computer-aided manufacturing (CAD/CAM) software, osteotomies may be planned and the jaws or other anatomic structures can be virtually repositioned in any plane of space [43-46].
3D Printing technology
3D printing is an advanced technology that was used for the first in dental implants and custom prosthetic devices [47]. It involves in a number of anatomical and surgical trainings [48]. 3D printing creates patient-specific models which have anatomical and/or tissue fidelity and has been recognized to assure more specific and safe surgeries for treatment, planning and simulation before oral surgery or orthognathic surgery [49]. It has also been used in other fields of dentistry, for instance, the individual occlusion could be accurately determined and treated with a unique splint fabricated using 3D printing [50]. 3D printing materials are powder based and vary depending on the nature (polymers, ceramics, plastics, resins, super alloys, stainless steel, and titanium) of the surgical application [51,52]. 3D printing technology could be useful for the improvement of surgical outcome and contribute to surgical planning by depicting precise personalized anatomy. However, some limitations of 3D printing technology, including long processing time, high cost, unsatisfactory mechanical properties and suboptimal accuracy might hamper the applications of this technology in daily clinical practice [53].
Discussion
The benefits of image-guided surgery have been reported in a number of studies, with improved results. In implantology the surgeon is no longer restricted to two-dimensional images therefore complications such as damage to the inferior dental nerve and perforations can be avoided. An accuracy of 1-2mm was reported by Wanschitz et al [54] in imageguided implantology using VISIT surgical navigation software. Template systems, designed to transfer a preoperatively defined implant position on to the surgical site, are also reported to produce accurate results. The use of a template eliminates errors in manual insertion and matches planning to prosthetic requirements. Implant operations can be simulated using Columbia Scientific SIM/Plant software. The three-dimensional implant positions can be transferred by computer to a milling machine, which mills the implant coordinate positions into the template. These positions will dictate the location, angle and depth of insertion of the implant.
Image guidance has also been used to improve the accuracy of osteotomies. SSN (Carl Zeiss, AG, Germany) was the first highly precise system to transfer laboratory-planned data concerning the repositioning osteotomy to the surgical site. It has been applied for repositioning osteotomies of the orbital walls, where it displays hidden levels of bone, which may not be visible with a bicoronal approach, and helps to navigate the complete structure. The Viewing Wand has been reported to achieve 2 mm accuracy in 40 operations including osteotomies and resection of tumours of the mid-facial area [55]. Satisfactory results have been recorded with the ARTMA head-mounted display system in secondary reconstruction of post-traumatic deformities of the zygoma [56]. This system can also be used to superimpose virtual osteotomy lines on the maxilla to guide the oscillating saw in orthognathic operations. Application of image guidance to surgical oncology is currently most successful in tumours associated with the bony structures of the maxillofacial region because of the lack of deformation of soft tissues. Wagner et al [57] used their guidance system (ARTMA) to navigate instruments to an osteosarcoma in the deep infraorbital region. Instatrak system has also been used to guide orbital dissection for removal of tumours from the orbit.
Future prospective
Computer-assisted navigation system has various areas for further evaluation and improvement. Reducing high costs of the system and time required for getting accustomed with it are important. The markers used for detecting geometrical center are prone to give false recordings due to sterilization and reuse, thus recognition of such flaws are required and amended time to time. Reconfiguration, rebooting and aborting navigation are few methods to solve, if errors are encountered, however, the correlation between these problems and model type has not been yet investigated.
Conclusion and Outlook
This review provides the reader with updated information and knowledge regarding the recent literature on surgical navigation. Navigation-assisted technology in craniomaxillofacial surgery increases reliability by facilitating correct safety margins and protecting vital structures. In addition, it increases the precision of radiotherapy planning and it facilitates the reconstruction process. However the costs for the navigation systems are very high, and the time for preparation for the surgery is longer in comparison with conventional technique. The navigation procedure gives more security, particularly in complex cases and also may result in a better clinical outcome for the patient. Further development of software programs may reduce the preoperative planning time and time spent during the operation.
Competing Interest
VK, SG, RKC, RKC, AG and SKC declare that they have no conflicts of interest directly relevant to the content of this article.
Author’s Contributions
SG, RKC, RKC and AG searched the literature and drafted the manuscript under the supervision of VK and SKC.




