Introduction
Acute renal failure in animals was found to be associated with adulterated pet foods containing wheat gluten contaminated with melamine [1]. Melamine is a chemical used for manufacturing of plastics and resins but also marketed as a fertilizer. However, in an attempt to increase the nitrogen content, melamine was found to be added into formula milk for children feeding [2,3]. It was well established that melamine added to food is mainly pure melamine with 1-2% cyanuric acid (cyanurate) contamination mainly coming from the production of melamine cyanurate [4]. In toxicological studies, melamine alone does not cause renal damage, whereas insoluble crystals are formed to cause renal tubular obstruction when combined with the co-contaminant cyanurate [1,5]. We have conducted our studies with similar dose as that would be seen in clinical situations. In the late 2008, melamine had caused the increase of renal stone incidences in infants and children in Mainland China [6,7]. Six deaths had been reported and more than 300,000 infants were found suffering from urinary tract ailments related to kidney stones, with 850 still being treated and 150 seriously ill [7]. In Hong Kong, over 40,000 children had been screened for the melamine-related stones, and 15 cases were reported positive [3]. Most children afflicted with melamine-related stones were described as asymptomatic until renal abnormalities, revealed by impaired renal function was so severe that melamine and its crystalline stone had done its damage on first clinical presentation [8]. Whilst most infants and children affected were less than 3-years old, the long-term effects and damage are largely unknown. There are numerous evidences that the presence of crystals, such as calcium oxalate mainly from diets, may cause renal cell and tissue damage [9] as well as elevating cytokines [10] and mediators of inflammation [1]. During such events, gene expression would be invariably altered to stimulate both physiological and pathological response. For melamine, many studies have been focused on patients’ histological samples [1,11]. Although the incidences of melamine-related stone are now infrequent, children may still suffer certain complications. The cytotoxicity of melamine and cyanurate is currently unknown at the level of gene expression, cytokine activation and inflammatory markers. The questions posed here are related to what effects does melamine and/or cyanurate have on cells in culture and at what concentrations and ratios? These are being addressed, in the present study, by using the human tubular cell line in an established transwell-insert model [12], whereby we can investigate forming crystals interacting with the apical cellular side which mimic the situations in the nephrons, in order to investigate the bio-mechanisms.
Materials and Methods
The two-compartment transwell culture system
The presence of melamine and cyanurate in the renal tubules demands investigation of the interaction of both the soluble form and the crystallized form to (i) address how they are absorbed and localized, (ii) also at what concentrations and (iii) understand the crystal-cell interaction with the current knowledge known about Calcium oxalate (CaOx) crystalcell interactions. The human WT 9-12 cell line of distal and proximal cortical tubule (CRL-2833; ATCC, Manassas, VA) was cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) (ATCC) with 10% fetal bovine serum (GIBCO, New York, NY) and in pre-coated culture flask with bovine collagen type I solution Sigma, St. Louis, MO) at 3.0 mg/ml concentration. This study is modeled on our previous transwell cell culture model for calcium oxalate [12] to investigate whether the melamine/cyanurate crystals could similarly work. The optimization study was performed on Human Kidney-2 human renal proximal tubular cells. For the subsequent experiments, given that much of the published data were on canines, we investigated the cell culture system on the human WT 9-12 cell line, which is clinically relevant. Intact cell monolayer were established on permeable polycarbonate transwell micro porous filter membranes (insert growth area 1.12 cm2 , 0.4 μm pore size) (Coster® Corning, New York, NY) according to the standard protocol described [12]. In brief, logarithmical cells were grown for 12-14 days to confluence from seeding density of 4.4 × 104 cells/insert. The two compartments of the culture system (apical and basal layers) were then separated by the intact monolayer reaching TEER approximately at 300 ohm cm2 . The integrity of cell monolayer was determined using Epithelial Tissue Voltammeter (World Precision Instruments, Hong Kong, China) and expressed as transepithelial electric resistance (TEER) values through the following calculation: (TEERsample – TEERblank) × surface area. The formation of tight junction was further confirmed by assessing the transepithelial flux of fluorescein isothiocynate (FITC)-dextran (10 KDa; Sigma) from the apical side to the basal side. Artificial urine (AU) was prepared as previously described [12]. Artificial urine was prepared daily. Analytical grade chemicals were dissolved in Milli-Q® water and pH was adjusted to 6.0 with hydrochloric acid. The final concentration of the major ions were calcium (6 mM), magnesium (3.0 mM), sodium (196 mM), potassium (82 mM), phosphate (23 mM), sulfate (20 mM), oxalate (1.2 mM) and citrate (2.2 mM). Urine was buffered with disodium phosphate (22 mM). Stock melamine solution (10 mM) was prepared by dissolving 1.26 gm melamine (C3H6N3, MW (126.1 g/mole), Sigma-Aldrich® ) in 1 l Milli-Q water. Stock cyanuric acid solution (10 mM) was prepared by dissolving 1.2908 gm cyanuric acid (C3H3N3O3, MW (19.1 g/mole), Sigma-Aldrich) in 1 l Milli-Q water. Crystallization in supersaturated AU with respect to melamine cyanurate was observed microscopically and measured in terms of turbidity at 405 nm.
Cytotoxicity and cytokine secretion
The concentrations of melamine, cyanurate and melamine/ cyanurate that will cause cell toxicity by cell death were investigated on flat-bottom 96-well culture microplate, by measuring Lactate Dehydrogenase (LDH) released in media, in order to guide the concentrations to be used in subsequent experiments. In addition, the clinical relevant ratio for melamine and cyanurate, suggested as 99:1 was also tested [4]. On the transwell experiments, to allow formation of new crystals directly on the apical surface of WT 9-12 cells, equal amount (each 250 μl) of appropriate dilutions of the melamine (50, 49.5, 25, 5 mmol/l concentrations) and cyanurate (50, 25, 5, 0.5 mmol/l concentrations) in AU were added separately into the insert well, and orbital shaking (10-minute) at 37 °C was immediately followed by recording on the TECAN SPECTRA Fluor Plus microplate reader (TECAN, Grodig, Austria). Parameters tested were melamine to cyanurate ratios at 1:1 (50:50, 25:25, 5:5 mmol/l concentrations) and 99:1 (49.5:0.5 mmol/l concentration). For checking the cellular viability immediately after exposure, agitated cells was trypsinized and counted using the ViCell counter (Beckman Coulter Miami, FL) using the trypan blue exclusion method. In parallel experiments, AU solutions containing melamine/cyanurate crystals were removed after agitation and cells, washed thrice with phosphate buffered saline (PBS), replaced with complete media and incubated for 24 hours to study the repairing of cell monolayer. Such post-exposure incubation was aimed to allow sufficient time for cellular response such as cell repair, gene expression, and cytokine secretion. At 24 hours, apical and basal media were collected for measurement for cytokine panel expression using eBioscience FlowCytomix T-helper (Th)1/Th2 11-plex and chemotaxis 6-plex assays (Bender MedSystems, Austria) with Cytomics FC500 Flow cytometer (Beckman Coulter, Miami) equipped with CXP software version 2.2. Cell nuclei were extracted for quantification of oxidative DNA damage using the highly sensitive 8-hydroxy-2’ -deoxyguanosine (8-OHdG) ELISA kit (Japan Institute for Control of Ageing, Shizuoka, Japan).
Human genome 44K gene expression microarray
Similarly to previous study with CaOx urolithiasis which constructed a expression profiling using cDNA microarray [13], we also investigate the gene expression when the cultured cells were exposed to melamine/cyanurate under normal physiological conditions and high levels using the 44K Whole Human Genome Oligo microarray on Agilent genomic platform. Total RNA of harvested cells were extracted by using RNeasy Mini kit (Qiagen, USA). The quality of RNA was assessed in terms of quantity (absorbance at 260 nm), purity (A260/A280 ratio of 1.9-21) and integrity (ratio of 28S rRNA to 18S RNA at 2:1 by using Agilent 2100 Bioanalyzer). All purified RNA samples were stored at -80 °C until assay. Four conditions with melamine, cyanurate alone and in mixture 50:50 and 49.5:0.5 (melamine: cyanurate) were tested against the AU control. The differentially regulated genes were then grouped into clusters based on their expression profiles and correlated with the cytokine expression panel work-up.
Statistical analysis
All assays were performed in duplicate for reproducibility. Differences between means were determined using ANOVA to compare mean differences with p < 0.05 considered statistically significant. All significant ANOVA test results were analyzed by the Dunnett multiple comparisons post-test. (GraphPad Prism Version 3.0 for Windows, GraphPad Software, San Diego, CA).
Results
Cytotoxic effects of melamine and cyanurate crystallization
Direct cytotoxic effects, as assessed by the release of LDH, was demonstrated by a mixture of melamine and cyanurate in a concentration-dependent manner, but neither by melamine nor cyanurate alone (Figure 1A). Approximately 60% of WT 9-12 cells were killed by mixture with 50 mM melamine and 50 mM cyanurate (1:1) (Figure 1B). On the cell monolayers with tight junctions, about 15-25% of the cell viability was affected immediately after 10-minute agitation incubation with melamine/cyanurate mixture at 1:1 ratio when compared with the AU control, irrespective to whatever the concentrations added (Figure 1C). Similar inhibitory effect on cell viability was demonstrated when tested with the clinical relevant 99:1 melamine to cyanurate ratio. However, neither cell repair nor further reduction of viable cell number was observed after 24 hours (Figure 1D). In addition to the microscopic observation showing no crystal adhesion on apical surface of cells, and observing no further cellular damage after the prolonged 24- hour incubation, results suggested that the cytotoxic effects caused by melamine/cyanurate crystals at 1:1 and 99:1 ratios were due to physical contact during agitation applied at the initial stage of experiment, and thus crystal uptake and endocytosis were not speculated.
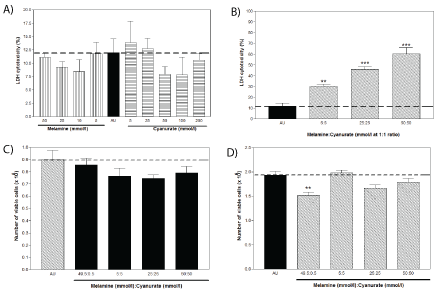
Figure 1: Cytotoxic effects of melamine cyanurate, as measured by LDH assay immediately after the 24-hour incubation with
Figure 1A: Melamine and Cyanurate alone.
Figure 1B: The mixture of Melamine and Cyanurate (** P<0.01; *** P<0.001). Whilst number of viable cells remained in the cell monolayer after.
Figure 1C: The 10-minute agitation with the mixture of Melamine and Cyanurate.
Figure 1D: 24-hour incubation as the crystal containing UA replaced by fresh culture media (** P<0.01).
Oxidative DNA damage induced by melamine and cyanurate crystallization
Oxidative stress was significantly (P<0.01) induced by melamine/cyanurate at 1:1 ratio, by 1.13 µg/ml 8-OHdG increase when compared with the AU control (Figure 2). However, such effect was not found in the culture with melamine/cyanurate at 99:1 ratio.
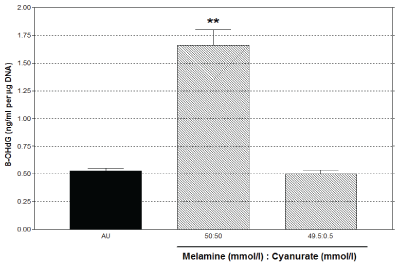
Figure 2: 8-OHdG as an oxidative stress marker was increased by melamine/cyanurate mixture at 1:1 ratio with 50 mmol/l melamine and 50 mmol/l cyanurate, as measured at 24 hours post exposure (** P<0.01).
Effect of melamine and cyanurate crystallization on cytokine secretions
Among the 16 Th1/Th2 cytokines and chemokines tested, baseline levels of IL-6, IL-8 and (monocyte chemotactic protein) MCP-1 were detected in the harvested media of the cultures at 24 hours, and higher levels were measured at the apical side. The secretion of IL-6, IL-8 and MCP-1 were increased by the melamine/cyanurate crystals in parallel with their cytotoxic effects (Figure 3A-C). Furthermore, IL-5 was not detected in the control media, whereas its secretion was also stimulated by the crystals (Figure 3D).
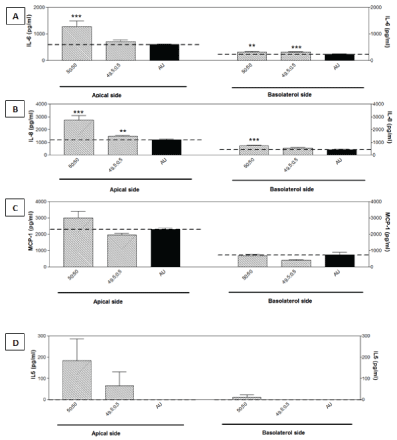
Figure 3: 8-OHdG as an oxidative stress marker was increased by melamine/cyanurate mixture at 1:1 ratio with 50 mmol/l melamine and 50 mmol/l cyanurate, as measured at 24 hours post exposure (** P<0.01).
Effect of melamine and cyanurate crystallization on the gene expression microarray
A total of 7,777 genes were up- or down-regulated in twofold magnitude in the cultured cells by either the 1:1 or 99:1 ratio of melamine to cyanurate. Cluster analysis showed that genes involved in inflammation, calcium-gated channels and zinc finger proteins were mainly affected. In the present study, we confined our results to the top 100 altered genes in which some common genes were altered by increasing the expression including the calcium binding and zinc finger proteins, amongst all tested conditions (Table 1). Genes for butyrophilin-like, claudin 19 and voltage-dependent calcium channel variant were remarkably down-regulated by the 99:1 ratio (Table 2), in contrast to other genes for leukocyteassociated immunoglobulin-like receptor, killer cell lectin-like receptor and synaptogyrin 1 were down-regulated by the 99:1 ratio (Table 3). However, up-regulation of certain cytokine genes including IL2, IL5, IL6, IL8 and chemokine-like factor were up-regulated by both ratios (Table 3), which correlated with the cytokine protein secretion examined in harvested media of culture and cytotoxic effects.
| Probe Name |
Description |
50:50:00 |
49.5:0.5 |
Melamine |
Cyanurate |
|
Fms-related tyrosine kinase 1 (FLT1) |
23 |
23 |
26 |
27 |
| up |
up |
up |
up |
| S100 calcium binding protein B (S100B) |
16 |
19 |
18 |
19 |
| up |
up |
up |
up |
|
CD48 molecule (CD48) |
11 |
11 |
12 |
13 |
| up |
up |
up |
up |
| Aquaporin 12A (AQP 12A) |
40 |
41 |
41 |
47 |
| up |
up |
up |
up |
| Zinc finger protein 645 (ZNF645) |
16 |
18 |
18 |
19 |
| up |
up |
up |
up |
| Zinc finger protein 516 (ZNF516) |
22 |
38 |
18 |
15 |
| up |
up |
up |
up |
| Lactate dehydrogenase A-like 6A (LDHL6A) |
19 |
21 |
6 |
19 |
|
up |
up |
up |
up |
Table 1: Expression of common genes altered to similar extent amongst all tested conditions as compared with the AU control.
| Probe Name |
Description |
50:50:00 |
49.5:0.5 |
Melamine |
Cyanurate |
|
A_23_P7412 |
Homo sapiens butyrophilin-like 8 (BTNL8), transcript variant 1, mRNA [NM_024850] |
No |
9397 |
2 |
No |
| effect |
down |
Up |
Effect |
|
A_23_P379054 |
Homo sapiens claudin 19 (CLDN19), mRNA [NM_148960] |
No |
1861 |
2.7 |
No |
| effect |
down |
down |
Effect |
|
A_24_P401686 |
Homo sapiens calcium channel, voltage-dependent, T type, alpha 1G subunit (CACNA1G), transcript variant 15, mRNA [NM_198397] |
No |
13 |
2.4 |
2.4 |
| effect |
down |
up |
Up |
| A_24_P820302 |
AF246221 transmembrane protein BRI {Homo sapiens} (exp=-1; wgp=0; cg=0), partial (43%) [THC2594845] |
12 |
20 |
8 |
9 |
| up |
up |
up |
Up |
| A_23_P209135 |
Homo sapiens leukocyte-associated immunoglobulin-like receptor 1 (LAIR1), transcript variant b, mRNA [NM_021706] |
454 |
2 |
3 |
2 |
| down |
up |
up |
Up |
|
A_23_P8723 |
Homo sapiens killer cell lectin-like receptor subfamily G, member 2, mRNA (cDNA clone MGC: 131846 IMAGE: 6139360), complete cds. [BC110858] |
75 |
No effect |
2 |
No effect |
| down |
up |
|
A_23_P348063 |
Homo sapiens synaptogyrin 1 (SYNGR1), transcript variant 1a, mRNA [NM_004711] |
55 |
No effect |
No effect |
No effect |
| down |
| A_24_P115967 |
Homo sapiens diacylglycerol lipase, alpha (DAGLA), mRNA [NM_006133] |
29 |
15 |
16 |
17 |
| up |
up |
up |
Up |
| A_32_P73452 |
Homo sapiens transmembrane protein 16H (TMEM16H), mRNA [NM_020959] |
27 |
8 |
6 |
9 |
|
up |
up |
up |
Up |
| Solute carrier family 24 (sodium/potassium/calcium exchanger) member 3 (SLC24A3) |
8.6 |
1.3 |
2.6 |
2.7 |
| down |
down |
down |
Down |
Table 2: Expression of genes, particularly altered by melamine:cyanurate at ratio 1:1 or 99:1 as compared with the AU control.
| Probe Name |
Description |
50:50:00 |
49.5:0.5 |
Melamine |
Cyanurate |
| A_23_P501713 |
Homo sapiens interleukin 1 family, member 10 (theta) (IL1F10), transcript variant 1, mRNA [NM_032556] |
1.6 |
2 |
3.1 |
2 |
| down |
down |
down |
down |
| A_23_P79398 |
Homo sapiens interleukin 1 receptor, type II (IL1R2), transcript variant 1, mRNA [NM_004633] |
4.2 |
3.1 |
4.6 |
3.5 |
| down |
down |
down |
down |
| A_23_P30115 |
Homo sapiens interleukin 2 (IL2), mRNA [NM_000586] |
8 |
9 |
9 |
9 |
| up |
up |
up |
up |
| A_23_P30122 |
Homo sapiens interleukin 2 (IL2), mRNA [NM_000586] |
1.7 |
1.9 |
1.4 |
2.2 |
| up |
up |
up |
up |
| A_24_P209047 |
Homo sapiens interleukin 5 (colony-stimulating factor, eosinophil) (IL5), mRNA [NM_000879] |
3.2 |
3.4 |
3.6 |
2.4 |
| up |
up |
up |
up |
| A_24_P935033 |
Homo sapiens alternatively spliced interleukin-6 receptor beta chain mRNA, partial cds. [U58146] |
3.9 |
4.3 |
2.8 |
2.7 |
| up |
up |
up |
up |
| A_32_P87013 |
Homo sapiens interleukin 8 (IL8), mRNA [NM_000584] |
22 |
13 |
14 |
25 |
| up |
up |
up |
up |
| A_24_P52733 |
Homo sapiens interleukin 10 receptor, alpha (IL10RA), mRNA [NM_001558] |
1.6 |
1.6 |
1.2 |
1.9 |
| up |
up |
up |
up |
|
A_23_P91943 |
Homo sapiens interleukin 12A (natural killer cell stimulatory factor 1, cytotoxic lymphocyte maturation factor 1, p35) (IL12A), mRNA [NM_000882] |
No effect |
1.5 |
2.3 |
No effect |
| down |
down |
|
A_23_P7560 |
Homo sapiens interleukin 12B (natural killer cell stimulatory factor 2, cytotoxic lymphocyte maturation factor 2, p40) (IL12B), mRNA [NM_002187] |
1.1 |
2.3 |
1.1 |
2.5 |
| up |
up |
up |
up |
|
A_23_P61057 |
Homo sapiens interleukin 16 (lymphocyte chemoattractant factor) (IL16), transcript variant 1, mRNA [NM_004513] |
3 |
3 |
3.5 |
3.6 |
| up |
up |
up |
up |
| A_23_P332820 |
Homo sapiens interleukin 17A (IL17A), mRNA [NM_002190] |
1.9 |
2 |
2 |
2.3 |
| Up |
up |
up |
Up |
| A_23_P167882 |
Homo sapiens interleukin 17F (IL17F), mRNA [NM_052872] |
2.3 |
4.6 |
6.5 |
3.8 |
| up |
up |
up |
Up |
| A_24_P215804 |
Homo sapiens chemokine-like factor (CKLF), transcript variant 1, mRNA [NM_016951] |
3 |
14 |
14 |
6 |
| up |
up |
up |
Up |
Table 3: Expression of interleukins/chemokines genes and their receptors caused by melamine: cyanurate at ratio 1:1 or 99:1 as compared with
the AU control.
Discussion
Our in-vitro studies of melamine crystals interaction with renal cells is modeled after our earlier work with calcium oxalate cell culture studies [12]. The study of crystal-cell interaction and its subsequent cell-damage and response is an active area of research in urolithiasis. Melamine cyanurate crystals mainly interacted with the cells physically whether it was 1:1 or 99:1 (clinical relevant). Pet food and milk product were mainly contaminated with melamine, and cyanurate, was a by-product contaminant with less than 1% [4] and this ratio of 99:1 is taken to be clinical relevant in our studies. Unlike CaOx crystals which have been shown to be actively taken up by renal tubular cells [12,14], melamine crystals caused physical damage to the cells and did not have any appearance of adherence to the cell surface - a necessary event before endocytosis occurs. LDH is a classic marker for cell-damage and subsequent oxidative stress on cells can be seen with melamine cyanurate present at 1:1 only and not at clinical relevant 99:1 ratios. Such cellular damage was also seen on human erythrocytes [15]. Oxidative stress is a cellular response to signal the immune system and cells in the vicinity of immediate danger and while our results does not show such stress at 99:1 melamine ratio, it does however predict that cells are either damaged due to high load of melamine, such as those infants continuously taking melamine-containing milk for prolonged periods. It is also suggested that low concentrations or acute dose of melamine would not damage the cells, however this needs further elucidation.
In an in-vivo situation such as physical injury, cells would call upon a localized inflammatory response and a host of cytokine factors to be released. Significant increase of IL-5, -6, -8 and MCP-1, suggests the cellular response to renal cell injury and promote the recruitment of host immune-competent cells such as neutrophils and monocytes. The presence of IL-5 and -6 also suggested a shift to Th2, which favors the humoral type of immune response. There have been reports of melamine inducing antibodies [16] and being able to raise antibodies in experimental models [17]. Hence, for cells to release mediators to support/enhance humoral type of immune response is consistent with the in-vivo studies in humans and animals. The overall microenvironment suggested that the crystals caused physical cell injury on the tubular cells to trigger proinflammatory reactions. The increase of cytokine levels at both apical and basolateral sides also suggested the interference of tight junctions on the cell monolayer.
Similar to a previous study [18] that looked at globally expressed genes during CaOx nephrolithiasis in rats, we report the gene expression microarray using whole genome scanning (Human genome 44K) when melamine/cyanurate is presented to the cells in culture. Invariably, during such studies using global scanning, there will be expression of several thousands of genes, cluster analysis of the top 100 genes expression showed that gene clusters involved in inflammation, calciumgated channels and zinc finger protein were the major clusters that were expressed. This is similar to the in-vivo animal model of global gene expression conducted on rats with CaOx urolithiasis [18], where similar clusters were affected. However, in our model, the magnitude of up/down regulation was several times higher and the genes shown in that study were distinctly different from those observed by us in the current study. This can be hypothesized that injury and uptake of CaOx urolithiasis is distinctly separate from melamine induced crystallization. The gene expression observed here, does give a snapshot of the possible types of cell responses to injury by melamine - mainly the inflammatory and Th2-humoral type of immune response. This is corroborated with the findings of cytokines profile secreted in the culture media. This could be the first report of such studies with melamine. As revealed in other studies with human kidney epithelial cells, genes for inducible cytokine subfamily were commonly involved in the crystal-induced cellular injury events [13]. At all tested conditions, we also found that the zinc finger proteins were universally up-regulated (Table 1), which is not surprising in that they have diverse functions such as DNA recognition, RNA packaging, regulation of apoptosis, protein folding and lipid binding [19]. Current results indicates that continual intake of melamine, even at safe levels (as prescribed by Hong Kong Centre for Health Protection and U.S. Food and Drug Administration) is suitable or not? However, to confirm which genes were affected and what proteins were affected would require further work and this is beyond the current scope of this study. In conclusion, melamine-crystal cell interaction was particularly different from other nephrolithiasis event (eg. CaOx and Calcium Phosphate (CaP)). Presence of clinically relevant levels of melamine brought about physical damage to cells with the concomitant release of cell injury markers, LDH and oxidative stress. The release of cytokine markers for inflammation and humoral immune response were evident and this is correlated to that found in other studies. Cluster analysis of the top-100 genes up/down regulated revealed that they were mainly protective-type, such as shutting calcium channels and inflammatory to recruit cells to remove damaged and dead cells. Overall, there was no evidence of long-term sustained damage of cells from melamine exposure, except of the physical effects, unlike that seen in CaOx urolithiasis, whereby cells internalized CaOx crystals and continue to accumulate and send inflammatory cytokines over a period of time until such CaOx within the cells were removed. From our current studies, we can now have a better understanding of the cellular effects of melamine, which is particular important in understanding the clinical manifestations of melamine related problem and also establishing a clinical management plan. Whether patients with more chronic melamine exposure will lead to prolong inflammatory reaction, and/or subsequently lead to long-term tubular-interstitial nephritis or even renal fibrosis is still unknown. Further studies are needed in these areas. The monitoring of some urine inflammatory markers may also be relevant in patients with prolonged high-level exposure and help to ascertain the extent of disease.
Acknowledgement and Funding Source
This project was funded by HKSAR Food and Health Bureau Grant M1-BS-07 awarded to Prof C.F. Ng at the Chinese University of Hong Kong. The funding sponsor was also responsible for quality assurance of the research.



