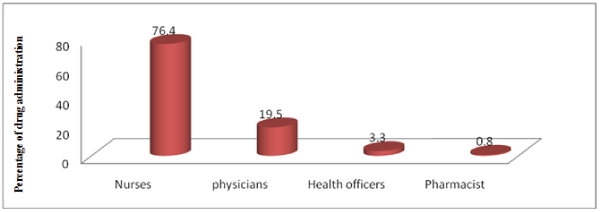
Figure 1: Distribution of health care team participated in medication preparation and administration in MKH from January 30-February 28, 2014

Sileshi Dubale1* Sultan Suleman2 Abdisa Gurmesa3
1Faculty of Public Health and Medical Sciences, Mettu University, Mettu, Ethiopia.*Corresponding author: Sileshi Dubale, Faculty of Public Health and Medical Sciences, Mettu University, Mettu, Ethiopia, Tel: 917806080; E-mail: sileshi.dubale@gmail.com
Background: Intravenous (IV) medication is an integral component of clinical care for hospitalized patient. Errors associated with IV medication can cause detrimental patient outcome. It affects patient’s life and can increase health care cost. It also involves high risk since it is delivered directly into the patient’s blood stream. As part of quality by design (QbD), Failure Mode Effect Analysis (FMEA) is a proactive tool used to analyze risks, identify failures and prioritize remedial measures. The major advantage of FMEA over other quality improvement schemes is the information gathered that makes it easy to identify the priorities of any actions required for improvement.
Objective: To assess prevalence, causes and severity of IV medication errors using FMEA in Mettu Karl Hospital, South West Ethiopia.
Method: Hospital based prospective cross sectional study was conducted for one month from January 30 to February 28, 2014 by using direct cross sectional observation of IV medication preparation and administration. Data was analyzed by using SPSS version 16.0 statistical software package. Frequencies of descriptive statistics were presented by using percentages and table. Binary, backward logistic regression analysis was performed to assess factors associated with IV medication failure mode to identify only significant root causes. We use R-soft ware for rating and categorizing of high Risk Priority Numbers along severity versus occurrence and delectability as failure mode effect analysis standard. Statistical significance was defined at a level of 0.05.
Result: From 123 IV medication preparation, 12 failure modes and 33 associated factors were identified. Aseptic technique was the most observed error, 106 (86.2%); Followed by 94 (76.45%) of wrong time and 92 (74.8%) of wrong rate. Human factor, 71 (57.7%) was the most contributing factor observed. Surgical ward (75.0%) and Gynecology ward (62.0%), were the first, and second wards in which IV medication failure mode was observed.
Conclusion: This study shows that there was a serious IV medication failure mode in each wards and needs prompt intervention.
IV medication process; failure mode; effect analysis.
ADES: Adverse Drug Events; AHEQ: Agency for Health Care Research and Quality; ASHP: American Society of Health System Pharmacist; DERS: Dose Error Reduction Soft ware; EPA: Ethiopian Pharmaceutical Association; EPHA: Ethiopian Public Health Association; FDA: Food and Drug Act; MKH: Mettu Karl Hospital; MEA: Failure Mode and Effects Analysis; IOM: institution of medicine; IPF: International Pharmaceutical Federation; IV: Intravenous; JCAHO: Joint Commission Accreditation of health care organization; LASA = Look Alike and Sound Alike; LOS: Length of Hospital Stay; MEPS: Medication Error Prioritization System; MOH: Ministry of Health; NCCMRP: National Coordination Council of Medication errors & Prevention; NGO: None Governmental Organization; NTI= Narrow Therapeutic Index; POC: Point of contact; RPN: Risk priority number; SPSS: Statistical package for Social Sciences; UK: United Kingdom; US: United States; USP: United States pharmacopoeia; WHO: World Health Organization.
Medication errors are any preventable events that may cause, or lead to inappropriate medication use or patient harm while the medication is in the control of the health care professional or patient. Of all medication errors, Intravenous (IV) medication error involves high risk since it is delivered directly into the patient’s blood stream. It is the most common types of injuries experienced by hospitalized patients. The issue received maximum attention in the immediate years after the institution of medicine (IOM) report was published in 1999.The published data demonstrates that approximately 5-10% of all hospital admissions are medicine (drug) related [1-4].
The source of medication errors is multidisciplinary and multi factorial. It usually occurs because of the breakdown in the systems that have been developed for handling and processing drugs. Some of the errors result in serious patient morbidity and mortality; compromise the confidence of patient in health care system, and lead to increased health care cost [5,6].
Hospitalized patients are subjected at least to one medication error per day with at least 1.5 million preventable each year. This reaction leads to an estimated $3.5 billion in addition to health care cost annually to hospitalized patient alone and the medication error is representing between 4th to 6th leading causes of death. Multidisciplinary team comprised of pharmacy, physician, nurses and biomedical engineers performed a failure mode and effect analysis (FMEA) to identify potential failure associated with IV pumps as a major academic medicine center; the study conducted in six hospital departments of three countries showed that 824 doses were prepared and 798 doses administered. The product was either not labeled or incorrectly labeled in 43%, 99%, and 20% of doses administered in the UK, German and French hospitals, respectively. At least one deviation from aseptic technique was observed among 100%, 58%, and 19% of cases in the three countries. In Australian hospital studies IV drug administrations have revealed a higher risk and severity of error than other medication administrations. A significant proportion of errors, suggest the main determinant factors are skill and knowledge deficiencies, with errors and severity reducing as clinical experience increases. A proportion of errors are also associated with routine violations which are likely to be learnt workplace behaviors; both areas suggest specific targets for intervention [7-10].
Failure mode and effect analysis (FMEA), as part of quality by design (QbD), is a proactive tool used to analyze risks, identify failures and prioritize remedial measures. To examine the hazards associated with the process of drug delivery to patient. Major advantage of FMEA over other quality improvement schemes is the unique information gathered that makes it easy to identify the priorities of any actions required for improvement, lowering the risk of the medication-use process [11-13]. Based on this ground, the primary objective of this study was to assess prevalence, causes, severity and effects of IV medication errors using failure mode effect analysis (FMEA) in Mettu Karl Hospital, South West Ethiopia.
The study was conducted at Mettu Karl Hospital south west Ethiopia. Mettu Hospital is Zonal Hospital found in Mettu town Ilu Ababora Zone Oromiya National state south west Ethiopia, 600 Kilometers far from Addis Abeba, the capital of the country. Mettu Hospital was established in the year 1948 GC and was renewed in 1985 by the Germany organization called “Menschen für Menschen”, and because of this its name was changed to Mettu Karl Hospital (MKH) to remember the head of the organization Karlheinz Böhm. MKH serves a total of 2.1 million people from the south west of the country including Oromiya, Gambella and Southern Nations & Nationalities and people national regions with total 199 beds [14]
Hospital based prospective cross sectional study was conducted for one month from January 30 to February 28, 2014.
Structured questionnaire standard formats prepared from previously done researches with some modification were used as the instrument of data collection and data was filled by direct observation of IV medication preparation and administration. Physician medication order sheets nurse medication administration records and condition of medication administered was observed on spot. Two nurse and two pharmacists who collect data were trained on how to collect data and failure modes and fill the questionnaire. One pharmacist and the investigator checked the completeness and consistency of the questionnaire, observation and the collected data.
Multi disciplinary team was formed from medical doctors, nurses, pharmacists. The team rated the severity of the primary dependent variables (IV medication errors) based on clinical judgment experience. Occurrence and observability of the failure mode were rated based on standard literature (based on the standard checklist as listed on operational definition). For the process failure and its causes, the corrective measures and point of control was planned during the data collection.
All the data entered, compiled and analyzed by using SPSS version 16.0. Frequencies of descriptive statistics were presented by using percentages and table. Backward binary logistic regression was conducted to identify factors associated with IV medication failure mode to identify only significant root causes. The investigator used R-soft ware for rating and categorizing of high risk RPN along severity versus occurrence and detectability as FMEA standard. Statistical significance was defined at a level of 0.05.
To ensure the validity and reliability of the data, training and demonstration was given to data collectors and supervisor by the principal investigator. Pretest of the data collection tool was done on 5% of patients on IV medication and fluid therapy. Data was checked for completeness, clarity and consistency by supervisor and principal investigator and any clue about the purpose of the study was not informed to health professionals since it may create awareness and deviate from routine IV medication process.
Prior to data collection, the researcher obtained official letter permission from the Jimma university post graduate and research office. The letter of permission was written to Mettu Karl Hospital requesting the cooperation of the hospital to allow the researcher to conduct the study on there. In addition, oral permission was obtained, and during data collection patients’ and health care professionals willingness was asked and data collection was done.
The figure -1 below shows that most of IV medications were prepared and administered by 94 (76.4%) nurses, and 1(0.80%) of pharmacists. This reveals that the pharmacists were the least health care team who participated in IV medication preparation and administration practice.

Figure 1: Distribution of health care team participated in medication preparation and administration in MKH from January 30-February 28, 2014
According to the table 1 below, antibiotics 58 (47.2%) were the most IV drugs preparation and administration error observed in Mettu Karl Hospital during the study period and followed by analgesics, 38 (30.9%) and cardiovascular, 8 (6.5%) drugs.
| S. No | Drug categories | Frequency | % |
| 1 | Antibiotics | 58 | 47.2 |
| 2 | Analgesics and anti inflammatory drugs | 38 | 30.9 |
| 3 | Cardiovascular and renal system drugs | 8 | 6.5 |
| 4 | CNS drugs | 7 | 5.6 |
| 5 | GI drugs | 5 | 4.1 |
| 6 | Endocrine drugs | 4 | 3.3 |
| 7 | Minerals/vitamins | 2 | 1.6 |
| 8 | Respiratory drugs | 1 | 0.8 |
| Total | 123 | 100 | |
Table 1: Distribution of categories of IV medication preparation and administration error observed in Mettu Karl Hospital from January 30 February 28, 2014
From 123 IV medication prepared and administered, a total of 70(56.9%) Failure Modes were observed (Figure 2).
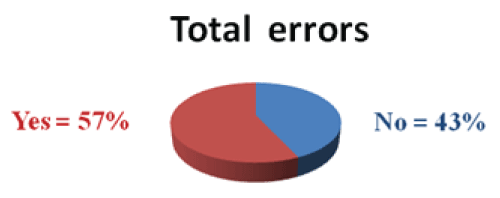
Figure 2: Percentages of total IV medication failure mode observed in Mettu Karl Hospital from January 30-February 28, 2014
This study identified that, from 12 failure modes of IV medication errors, aseptic technique error 106 (86.2%), wrong time error 94 (76.4%) and wrong rate errors 92 (74.8%) were the first, second and third most observed failure modes. Under dosing errors 23 (18.7%) is the least failure mode observed. Concerning the severity of the error, the most fatal errors were observed related with wrong rate 29 (23.6%), wrong time 15 (12.2%) and wrong preparation technique 14 (11.4%) [16,17] (Table 2).
| S.N | Failure Modes | IV medication Error observed | ||||
| No | Yes | |||||
| N% | NS | S | F | Total | ||
| (N/%) | (N/%) | (N/%) | N (%) | |||
| 1 | Aseptic technique error | 17(13.8) | 38(30.9) | 58(47.2) | 10(8.1) | 106(86.2) |
| 2 | Wrong time | 29(23.6) | 34(27.6) | 45(36.6) | 15(12.2) | 94(76.4) |
| 3 | Wrong rate | 31(25.2) | 23(18.7) | 40(32.5) | 29(23.6) | 92(74.8) |
| 4 | Wrong calculation | 37(30.1) | 37(30.0) | 36(29.3) | 13(10.6) | 86(69.9) |
| 5 | Wrong preparation techniques | 41(33.3) | 23(18.7) | 45(36.6) | 14(11.4) | 82(66.7) |
| 6 | Wrong diluents type &/or volume | 49(39.8) | 32(26.0) | 37(30.1) | 5(4.1) | 74(60.2) |
| 7 | Over dosing error | 60(48.8) | 13(10.6) | 41(33.3) | 9(7.3) | 63(51.2) |
| 8 | Drug omission error | 61(49.6) | 23(18.7) | 36(29.3) | 3(2.4) | 62(50.4) |
| 9 | Drug compatible error | 73(59.3) | 13(10.6) | 32(26.0) | 5(4.1) | 50(40.7) |
| 10 | Unauthorized drug given | 86(69.9) | 15(12.2) | 14(11.4) | 8(6.5) | 37(30.1) |
| 11 | Deteriorated drug given | 89(72.4) | 10(8.0) | 20(16.3) | 4(3.3) | 34(27.6) |
| 12 | Under dosing error | 72(58.5) | 23(18.7) | 26(21.1) | 2(1.6) | 23(18.7) |
Table 2: Distributions and severity of IV medication process failure mode identified in Mettu Karl Hospital from January 30 February 28, 2014
Key: NS: No significant (mild error); S: Significant (moderate to severe error); F: Fatal (life threatening error); T: Total error observed; N: Number observed.
From 123 IV medications failure mode observed, surgical ward 15 from 20 observations (75%), gynecology ward 18 from 29 observations (62%), medical ward 19 from 34 observations (55.9%) were the first, second and third ward error observed and ICU 6 from 11 observations (54.5%) and pediatrics ward 12 from 19 observations (41.4%) were the forth and fifth wards at which IV medication failure mode observed. Regarding the time at which IV medication failure mode observed, regular working time 38 from 63 (31.0%) and work shifting time 8 from 14 (57.1%), Weekend working time 6 from 11 (54.6%) were the first, second and third time at which IV medication failure mode observed and night duty were 17 from 35 (48.6%) were the least time at which IV medication failure mode observed [18,19].
From the total of IV medication process cycle observed, more than half 64(52%) of IV medication failure mode were observed at medication/drug administration process cycle and drug preparation process cycle 41(33%) was the second process cycle at which the failure mode was observed during the study period (Figure 3).
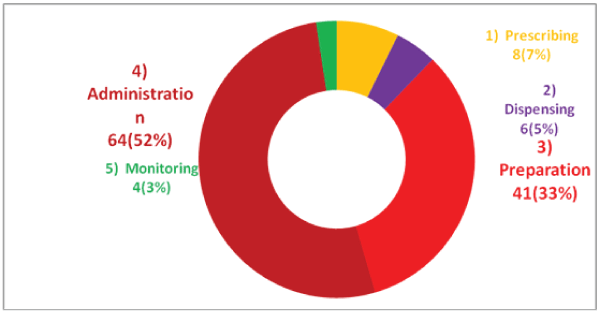
Figure 3: Percentage of the process cycle at which IV medication Failure Mode observed in Mettu Karl Hospital from January 30-February 28, 2014
Based on risk priority number, from 12 IV medication failure modes identified aseptic technique error (RPN=125), over dosing error (RPN=100) and wrong preparation errors (RPN=80) were the top three prioritized failure modes (Table 3).
Table 3: Examples of the serious IV medication process failure mode, potential cause, potential effect and severity observed and corrective action to be taken in study wards of MKH from January 30 February 28, 2014.
Key: D: Delectability; O: Observablity; S: severity: RPN: Risk priority number; HS: Hazards score; POC: Person of contact or process control.
According to the figure below the severity was analyzed by plotting the RPNs of higher risk failure modes in a priority matrix which is a graph divided into four colored areas reflecting different levels of priority for action. Area 1 (red) urgent action required; area 2 (orange) a prompt action required; area 3 (yellow) scheduled actions required; area 4 (green) only monitoring required [12,20-22].
The priority matrix gave each of the errors, graphical evidence of which steps, in the complex process of administering drugs, more urgently needed corrective action to reduce the risk of failures. Aseptic technique error (RPN=125), Wrong time error (RPN=100), Wrong rate error (RPN= 80) (Figure 4).

Figure 4: Priority matrix, plotting severity against probability (O×D) of IV medication failure modes from January 30-February 28, 2014 in Mettu Karl Hospital.
The leading cause of IV medication preparation and administration failure mode in this study area was human factor 71 (57.7%), (both patient related 96 (78.0) and health care team related 46 (37.4%) and followed by system or procedure related factors 62 (50.4%), and environmental factor 42 (34.1%). Product /drug related factors 39 (31.7%) were the least to cause IV medication errors. For each person the outcome was whether the patients developed IV medication errors or not. From the type of IV medication errors we consider Aseptic technique errors, Wrong time, Wrong rate, Wrong diluents, Wrong preparation technique and Wrong calculation failure modes. Aseptic technique error was highly associated with availability of the product (p-value=0.011), and pharmaceutical distribution system of the Hospital (p-value=0.049). Drug distribution system of the Hospital was also highly associated with wrong time error (P-value=0.004). Availability (p-value=0.011) and affordability of the drug (p-value=0.006) are highly significant to cause wrong time errors. And wrong rate error was significantly associated with experience of health care team (p-value =0.046) and lack of technology (p-value=0.046).
The odd of having drug storage problem causes aseptic technique errors is 12.866 times more than no drug storage problem. Availability, affordability and drug distribution have a significant relationship with the risk of developing wrong time error. The odds of having drug distribution system problem causes wrong time error 0.072 times than those having no drug distribution system problem. Lack of technology, duty time, fatigue and lack of experience have a significant relationship with the risk of developing wrong rate error. All the confidence intervals constructed did not include one number this support the relationship between the predictor variables and the outcome variable. The odds of having lack of technology cause wrong rate error 9.570 times than those having no lack of technology. Noisy area, fatigue, verbal ordering of the drug and lack of documentation have a significant relationship with the risk of developing wrong calculation. Negligence has no significant relationship with the risk of developing wrong calculation. The odd of having noisy area during medication preparation and administration causes wrong calculation 9.690 times than having no noisy area. Noisy area, duty time, knowledge and miscommunication have no significant relationship with wrong preparation technique. Lacks of technology, affordability of the drug to buy, experience and availability of the drug and other products in the Hospital have a significant relationship with wrong diluents error.
From 123 IV medication preparation and administration observed, there were a total of (56.9%) failures mode observed. It was nearly equal with IV medication error observed in three Brazilian hospital during 2006 (57.8%) and less than the study of two major teaching hospitals in Sydney Hospitals, Australia (69.7%). This difference is maybe because involvement of much specialty wards, long time study period and large sample size in their Hospital [4].
The finding was greater than IV medication error observed in Air Bus hospital, Denmark, in 2005(43%), and IV medication errors in Jimma University specialized Hospital ICU (51.8%). The discrepancy is may be due to the fact that, the design and the participant wards difference [23,24]. From 12 failure modes, aseptic technique 86.2%, wrong time 76.4%, wrong rate 74.8%, and wrong calculation 69.9% were among higher prevalence failure modes identified. In contrast, with the study of three European countries (UK, Germany and France) there was wrong diluents error 1%, 49%, 18%, respectively, and wrong rate error was 49%, 21%, 5%, respectively in each country this difference is may be due to long time study period multi center and multipurpose Hospital involvement of the three countries. At least one aseptic technique error observed was in the UK (100%), Germany (58%) and France (19%) was related with our study [10].
It was quietly different from IV medication error type identified in Air Bus hospital, Denmark, in 2005, the most common types of error throughout the medication process were: lack of drug form, unordered drug, omission of drug/dose, and lack of identity control, this discrepancy may be due to the fact that the difference in study design tools [23]. Common medication administration errors in the ICU of JUSH was attributed to wrong timing (30.3%), dose omission (29.0%) and missed doses (18.3%) among others, this difference is due to difference in the study ward. Errors associated with antibiotics were high in medication administration errors in Mettu Karl Hospital 58(47.2%) was the first category at which failure mode observed, almost similar with Jimma University Specialized Hospital ICU (36.7%) and the two major teaching hospitals of Sydney (two-thirds) [4,24]. Regarding fatality of each categories of IV medication failure mode, there was about 23.6% of wrong rate error, 11.4% wrong preparation technique and 10.6% wrong calculation errors are among fatal error observed. According to Besançon University Hospital (France) study, no potential fatal errors were observed, 10% were estimated as potentially life-threatening and 26% potentially significant. German nonUniversity hospital study only potentially severe errors 3%, potentially moderate errors 31% and potentially minor errors 13% identified. The differences may be due to utilization of advanced technologies for IV medication administration and dose calculation like smart pump infuser and computer based dose calculation [25,26]. Surgical ward (75%) and gynecology wards (62%) were the most wards in which higher prevalence of IV medication failure mode observed. Air Bus study show that six of 43 (14%) in the medical department and 1 of 56 (2%) in the surgical ward this is may be due to the fact that IV medication is administered for all patients in admitted in surgical ward in our case. According to Besançon University Hospital (France) 21% and 42% respectively of medication administration error was e life threatening and significant in ICU. May be due to the presence of different types of ICU based on specialty like Geriatric Unit, Cardiovascular and Thoracic Surgery Unit in their Hospitals [25,23]. From total IV medication observed, most of the failure modes were observed at regular working time (31.0%). However, according Jalan University, Malaysian study in 2013 administration at 8.00am (work shifting time) was significantly associated with a higher rate of medication error [6]. This discrepancy was may be due to more date was collected during day time in our case.
From total of IV medication process cycle observed, greater than half (52%) of IV medication failure mode was observed at medication/drug administration process cycle and drug preparation process cycle (33%) was the second step at which the failure mode observed during the study period. According to Air Bus study, the frequency of medication errors was observed highly at level of ordering (39%), transcription (56%), dispensing (41%) [23]. This differences may be because of study design difference, in our case the observation was limited only on medication preparation and administration and physicians order sheet and nursing medication chart at patient bed side.
Based on detectability, occurrence and severity rating, during the study period, the priority matrix gave each of the errors, graphical evidence of which steps, in the complex process of administering drugs using R-soft ware, more urgently needed corrective action to reduce the risk of failures. Aseptic technique error (RPN=125), Wrong time error (RPN=100), Wrong rate error (RPN= 80), need urgent intervention
The priority matrix of this study identified that from 12 of IV medication failure modes, six of them need urgent intervention (Aseptic technique error, Wrong time error, Wrong rate error, Wrong calculation error, Wrong preparation techniques error, Wrong diluents). With high RPN 60-125, it is similar in number with risk priority number identified in Padua University Hospital of pediatric ward in Italy, but it was different in type of IV medication failure modes identified almost related with wrong calculation (wrong calculation for the dose of bolus and infusion, wrong calculation for the rate, transcription error in new therapy, failure to notify time of infusion therapy start and failure to identify the diluted drug before storing in the refrigerator. According to Alan Polnariev, the Medication Error Prioritization System (MEPS) study in the June 2014, all medication errors reported by pharmacy staff using the online data-base were categorized into one of three classes based on a severity scale, scores with a value above 20 are classified as high priority in contrast to our study the RPN value above 60 categorized as high risk. It was almost similar by observation of, no harm to the patient. The difference was due to difference in study participant and tools of assessment (pediatrics and medication calculation related tools in case of Lago P. et al) and difference in priority seting standard in case of Polnariev A et al. [12,21]. From 12 failure modes and 33 associated factors identified, the leading cause of IV medication preparation and administration errors in this study area was human factor (57.7%), (both patient related (78.0) and health care team related (37.4%) and followed by system or procedure related factors (50.4%), and environmental factor was the least to cause IV medication errors. Aseptic technique error was highly associated with availability of the product, and pharmaceutical distribution system of the Hospital. Drug distribution system of the Hospital was also highly associated with wrong time error. Availability and affordability of the drug are highly significant to cause wrong time errors. And wrong rate error was significantly associated with experience of health care team and lack of technology. Similar study show that, nurse workload and incomplete or illegible prescription were two independent risk factors of medication and administration error ETsso et al study and no statistical difference between the error rate per patient in the wards in case of LISBY M et al. [25,23].
This study revealed that there was high prevalence of IV medication failure modes with multiple factors; greater than half of patient ware at least exposed to one IV medication error per day. Antibiotics and Analgesics were most commonly encountered drug categories in medication preparation and administration failure modes
Most of the failure modes were identified with high risk priority number and serious to cause patient harm. Human related factors and system related factors were the most contributing factors to cause patients’ harm in the Hospital. Based on the priority matrix, from 12 IV medication identified 6 of them should be intervened with in short period of time. Finally the study showed that there were serious IV medication and administration errors and each of them were caused by several factors identified as listed in the result. A multidisciplinary approach to solve the problem of medication errors should be practiced. Prompt intervention is very important to reduce the risk of IV medication error identified and prioritized
Mettu Karl hospital should increase the availability of drugs and other pharmaceutical products, and staffing of health care teams.
In-service training should be given to health professionals who are directly or indirectly involved in IV medication preparation and administration practices. Inappropriate drug storage at patient bed side should be avoided and central storage and preparation of IV medication should be adopted and pharmaceutical care services should be practiced in the Hospital wards. Patients who cannot afford to buy drugs should get free serves in Mettu Karl Hospital and/or patient should get drug by affordable cost. Different professional standards like nursing and pharmacy practice standards, save and conducive environment for IV medication preparation and short duty time should be arranged for staff involving IV medication preparation and administration practices in the hospital.
Hospital should allocate budget for maintenances, infrastructures and different equipments as well as utilization of new technologies like smart pump infuser. Guideline on how to prepare and administer IV medication should be prepared by Hospital managements and adhered during medication preparation and administration by health care teams. Control and reporting mechanism of IV medication error should be practiced in the Mettu Karl Hospitals.
Ministry of health Regional health bureau and different non governmental bodies who work in patient safety issues should focus and work on prevention of medication errors and patient harm. Further study should be conducted in large scale across the country to identify more problems and to implement control mechanism.
The authors declare that they have no competing interests
First Author was involved in the selection of the study topic selection, data collection, analysis, and preparation of the manuscript. Second Author was involved in topic selection, advising, analysis and reviewing of the manuscript while third Author was involved in advising data analysis reviewing of the manuscript. All the authors have read and approved the manuscript.
We extend our gratitude to Jimma University for funding to conduct this study and to Mettu Karl Hospital for allowing and hosting us for data collection.
Download Provisional PDF Here
Article Type: Research Article
Citation: Dubale S, Suleman S, Gurmesa A (2017) Failure Mode and Effect Analysis (FMEA) of IVMedication Process in Mettu Karl Hospital, Mettu town, Oromiya Regional State, South West Ethiopia Clin Res Open Access 3(1): doi http://dx.doi.org/10.16966/2469-6714.118
Copyright: © 2017 Dubale S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access