Abstract
Importance: Chronic Fatigue Syndrome (CFS) is a chronic disease resulting in considerable and widespread cognitive deficits. Accurate and
accessible measurement of the extent and nature of these deficits can aid healthcare providers and researchers in the diagnosis of this condition,
choosing interventions and tracking treatment effects. Here, we present a case of a middle-aged man diagnosed with CFS which began following
a typical viral illness.
Observations: LORETA source density measures of surface EEG connectivity at baseline were performed on 3 minutes of eyes closed deartifacted19-channel
qEEG. The techniques used to analyze the data are described along with the hypothesized effects of the deregulation found
in this data set. Nearly all (>90%) patients with CFS complain of cognitive deficits such as slow thinking, difficulty in reading comprehension,
reduced learning and memory abilities and an overall feeling of being in a “fog.”Therefore, impairment may be seen in deregulated connections
with other regions (functional connectivity); this functional impairment may serve as one cause of the cognitive decline in CFS. Here, the
functional connectivity networks of this patient were sufficiently deregulated to cause the symptoms listed above.
Conclusions and significance: This case report increased our understanding of CFS from the perspective of brain functional networks by
offering some possible explanations for cognitive deficits in patients with CFS. There are only a few reports of using source density analysis or
qEEG connectivity analysis for cognitive deficits in CFS. While no absolute threshold exists to advise the physician as to when to conduct such
analyses, the basis of his or her decision whether or not to use these tools should be a function of clinical judgment and experience. These
analyses may potentially aid in clinical diagnosis, symptom management, treatment response and can alert the physician as to when intervention
may be warranted.
Keywords
qEEG; LORETA; Source analysis; Chronic fatigue syndrome; Phase lag; Phase shift; Phase reset; Phase; Coherence; Cognitive
impairment
Introduction
Chronic fatigue syndrome (CFS) is a major health condition that
is associated with numerous body system dysregulation, including
substantial cognitive deficits in more than 90% of patients, affecting over
17 million people worldwide, and about 1 million people in the United
States alone [1-3]. As such, CFS represents a significant economic burden
to society, greatly decreased quality of life for patients and considerable
morbidity [4]. This contrasts with a relatively sparse neuropsychological
research base for this disorder, documenting only modest levels of
cognitive deficits [5-7] through neuropsychological testing [6] and other
types of neuroimaging techniques [8]. These testing and imaging reports,
however, do indicate some deficits involving attention and concentration,
memory, and information processing speed [6,8-10], though patients tend
to report much higher levels and more varied types of impairment than is
reported in research literature.
Measures which address neural dynamics with a high time resolution
can be co-registered to the MRI and PET and SPECT images, providing
a millisecond analysis of brain neural activity, which can be easily
compared to studies using other modalities. qEEG/LORETA measures
are the only such modalities which capture this millisecond time scale
activity [11-13]. A much more thorough understanding of cognitive
deficits in neurocognitive disorders will require the depiction of the rapid
coupling that takes place in neural oscillations on a millisecond timescale
[14,15]. Using LORETA (source analysis of the qEEG signal) [16], the
spatial resolution is about 1-3 cm and in qEEG the spatial resolution
is about 1 cubic centimeter [17]. The maximum spatial resolution of
fMRI is a little less than 1 cm, which is only slightly higher than qEEG
or LORETA [16,18,19]. The advantage of using qEEG or LORETA is the
greatly decreased cost and the considerably superior temporal resolution
[11,18,20]. qEEG measures for connectivity analysis lack the spatial
resolution of LORETA source analysis, but have the same temporal
resolution, providing inexpensive and easy to interpret brain function
[20].
qEEG and eLORETA measures [21-24] have found significantly
deregulated delta sources (1-4 Hz) in widespread bi-lateral portions
of the frontal lobe and limbic lobe regions as well as deregulated beta
activity in posterior parietal regions in CFS. The co-occurrence of cortical
hypoactivation in these brain regions provides empirical evidence for a
neurobiological basis pertaining to patient symptomology including
impairment in higher brain functions. Research [22] has found surface
qEEG effects of peak alpha frequency (PAF), computed within the 8-12 Hz
frequency band based on each participant’s EEG indicating significantly
decreased PAF over 58% of the entire cortex in patients with CFS when
compared to controls (11 electrode sites, p < 0.05). These findings are
consistent with previous reports of reduced efficiency of thalamocortical
connections in cognitive impairment [25-30] and suggest that EEGPAF
measurement may have both diagnostic and prognostic value
in patients [31,32]. There is now a need to better capture dynamic
relationships to understand a number of cognitive domains where CFS
deficits have been found.
The human brain creates meaning, cognition and perception by way
of continuous information flow which changes within milliseconds, and
then evaluates matches and mismatches of those expectations against
current sensory information available [13,15,33,34]. This process, based
on prior experience (memories, learning history) and genetics, creates
the expectations. Attention and arousal are produced when a novel
event occur followed by excitation of the reticular formation which then
promotes excitatory activity in the cortex [35,36]. During attention, the
brain first filters out irrelevant information, then continues to process the
relevant information [37-39]. This type of reductive decision making is
essential to operate in the world. When this process is compromised in
disease or injury, attention deficits, anxiety problems, and other negative
states are created due to limited resource allocation efficiency. The
switching dynamics-known as phase reset--phase shift and phase lock of
rhythm patterns--form homeostasis to support normal brain function.
Instabilities or disruptions in the homeostasis, in this system have been
associated with pathology such as autism [40], epilepsy [41], cognitive
deficits [42], and traumatic brain injury [43].
A review of the literature demonstrates that this system, known as
EEG coherence (an overall functional connectivity measure) is related to
a mixture of phase locking interrupted by phase shifts in the spontaneous
EEG, operating through phase reset synchronization mechanisms
(phase shift and phase lock duration) [44-49]. These fundamental brain
mechanisms operate continually in flux at various frequencies across
nodes of networks during the execution of any behavioral or cognitive
task. Canavier and colleagues [50,51] demonstrate that phase-reset (phase
lock followed by phase shift) represents our thoughts, feelings, and actions
via coordination between mutually connected, phase coupled, brain
regions. More importantly, Frey and colleagues [51] demonstrate the
effect of phase reset on human cognition, especially in clinical disorders.
Phase reset (phase shift and phase lock) are therefore fundamental brain
mechanisms which underlie the physiological basis of the cycle described
above.
Phase reset is made up of the two main physiological processes which
make up phase reset [29,51]. Phase lock synchronizes millions of neurons
across domains or networks within periods of 100-600 milliseconds.
Phase shift then releases the locked synchronization and recruits a new
set of neurons [17,40,51,52]. Phase shift allocates all available neurons
for performing a given function and typically varies between 40 and
80 milliseconds in length. Longer phase lock periods have been found
to be inversely correlated with intelligence due to the brief increase in
committed neurons which create a momentary reduction in neurons,
and phase shift has been shown to positively correlate with intelligence
[52,53]. The following case report explores several of the issues reviewed
above using qEEG/LORETA with a patient with CFS. We hypothesized
that we would see overall, global deregulation, especially in the frontal,
frontal-parietal and temporal lobes, as well as in limbic centers such as the
anterior cingulate, along with disrupted surface connectivity.
Report of a Case
A 43-year-old male patient diagnosed with prior CFS was assessed
with qEEG/LORETA as his request. This individual had been diagnosed
with CFS by his physician, using the DePaul Symptom Inventory, and
met the Canadian Clinical Case definition. Individuals with CFS typically
report multiple cognitive complaints including many types of memory
issues (working memory, metamemory, explicit memory, long-term
storage and retrieval, etc.), decreased learning ability, slowed thought,
difficulty with navigation (many cannot drive an automobile), problems
with concentration and attention and general, overall decreased alertness
known as “cognitive fog.”This individual had these types of neurocognitive
impairments, along with other classic CFS symptoms such as postexertional
malaise and sleep impairment. IRB approval from DePaul
University was obtained to do this study.
Three minutes of eyes-closed resting EEG was recorded with Neuroguide
software (version 2.7.4) with a 19-channel Electro-cap (Electro-Cap
International, Easton, OH) positioned according to the International
10/20 system of electrode placement. Electrodes were referenced to linked
ears with impedances below 5k ohms and the linked ears montage was
used in data analysis. Data acquisition was obtained using a Discovery
24E amplifier (BrainMaster Technologies, Bedford, OH) at 256Hz sample
rate with a 60Hz low-pass filter. Offline analysis was then conducted
with Neuroguide software using automated detection and rejection of
epochs containing any muscle, drowsiness, and movement artifact after
“eyeballing” the data set to look for any type of gross abnormalities in the
data. Raw data then was re-examined after the automatic processing of
artifact removal. Two minutes and 37-seconds of artifact-free data were
selected from the record, exceeding the 40-second minimum needed to
obtain a high reliability coefficient of 0.90. Neuroguide was also used to
compute reliability coefficients for each electrode site within each record;
split half and test-retest reliability coefficients were kept above 0.95. After
examining qEEG measures, we then computed LORETA to localize deeper
cortical sources of scalp EEG activity with comparison to qEEG norms.
This case report outlines the cortical source effects for CFS. First, the
LORETA source analysis illustrates abnormal source density at 2Hz (delta)
(Figure 1), indicating an overall decrease of cortical activation associated
with large-scale cortical integration which affects attention, arousal and
more recently, greater psychological pain orthogonal to depression [54]
operating through thalamocortical networks. It is important to note that all
levels of consciousness (including sleep/coma) are comprised during slow
cortical potentials (we found significant decreases in all rhythms, 1-30Hz
but present only delta 2Hz and beta 12-15Hz here), creating overall deficits
in arousal, attention, processing speed, integration and other cognitive
processes [11,30]. We hypothesize that this overall decreased arousal
may contribute to the often-reported state of brain fog in CFS. Second,
the surface qEEG connectivity analysis illustrates a higher rate of phase
resets per second than normal in beta (Figure 2) producing information
transfer that is deregulated within neocortical local and long-distance
circuits. For phase shift and phase lock duration, when both of these
processes are significantly shorter, fewer neuronal resources are allocated
for subsequent phase lock periods. These processes were authenticated in
this data (Figure 2); i.e., too-fast phase shift followed by too-short phase
lock which has been shown elsewhere to lead to inefficiency as a function
of time.
Conclusions
Our case study confirmed the pattern of dysregulation in the cortex
reviewed in the introduction. Furthermore, since both periods of phase
shift/lock durations were found to be significantly shorter, that might
contribute to an increased rate of phase reset, also seen in our data. Phase
reset deregulation--phase locking periods being too brief and phase reset
happening too often—appear to be consistent with the associated lower
rate of information processing and reaction times found in the ME and
CFS literature. These deregulated states represent the brain during nonoptimal
functioning, rendering it inefficient for most types of information
processing functioning, whether it is executive functioning, memory,
perceptual reasoning or information processing speed. When phase lock is
significantly less than normal, as in this data set, the ability of the brain to
sustain commitment of resources to mediate different functions is severely
compromised. Phase shift duration in this data is also hypoactive, meaning
that significantly less neurons are being recruited to perform a function
than normal. The results here indicate slowed verbal comprehension,
executive functions, perceptual reasoning, processing speed and memory,
the sum total of which is known as cognitive impairment.
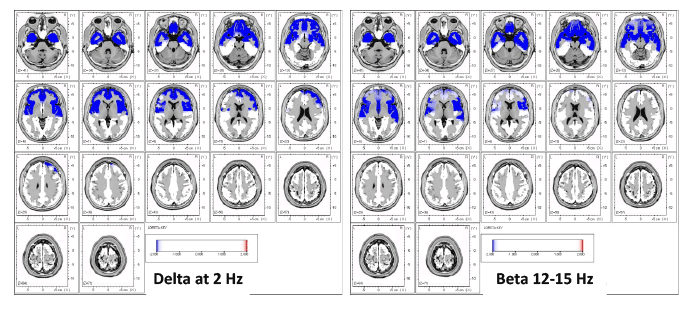
Figure 1: Results of LORETA current source density in a case with CFS showing widespread decreased current density for delta at 2 Hz and beta (12-
15 Hz) demonstrating a global reduction in brain functioning (blue). The higher frequencies (beta) have been shown to be a function of delta frequencies.
In other words, local oscillations are under constant influence of global brain dynamics (Buzsaki, 2006).
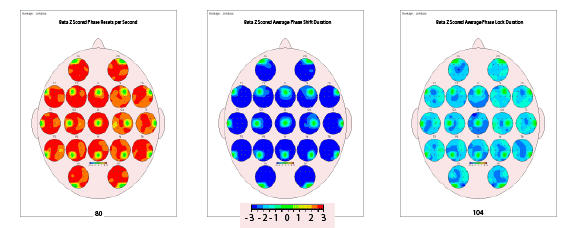
Figure 2: Surface qEEG connectivity topographs showing 3 aspects of phase reset in a case with CFS: z-scored resets per second, phase shift
duration and phase lock duration. All three metrics were found to be significantly deviant from normal in beta (12-25 Hz). Red color indicates 3 standard
deviations above, blue color indicates 3 standard deviations below. Rapid phase reset combined with shortened phase shifting and phase locking
periods demonstrates a global decrease in neuronal resource allocation and inefficient information processing speed
Patients with CFS often report premorbid functioning to be much more
efficient, quicker, and complex than their functioning after becoming
ill. Unfortunately, due to the sharp contrast between patient report and
research findings, there is a pervasive lack of consistency in measurement
of cognitive impairment in this population. This situation has stalled
forward movement by fostering the incorrect notion that cognitive deficits
faced by patients with CFS are created by psychological and emotional
factors and have little or no physiological pathology [55]. Using qEEG/
LORETA methods may provide a vehicle whereby the patients’ symptoms
and complaints can be validated by analyzing both surface and deeper
electric current sources occurring within the brain in 3 dimensions [56-
58]. This study involved only one patient, so until it is replicated with
larger samples, the results need to be considered preliminary.
Acknowledgement
Our thanks to Linda Clark for generously providing us financial
support
Article Information
Article Type: Case Report
Citation: Zinn ML, Zinn MA, Jason LA (2016)
qEEG / LORETA in Assessment of Neurocognitive
Impairment in a Patient with Chronic Fatigue
Syndrome: A Case Report. Clin Res Open Access 2(1): doi
http://dx.doi.org/10.16966/2469-6714.110
Copyright:© 2016 Zinn ML, et al. This is an
open-access article distributed under the terms
of the Creative Commons Attribution License,
which permits unrestricted use, distribution, and
reproduction in any medium, provided the original
author and source are credited.
Publication history:
Received date: 14 Jan 2016
Accepted date: 27
Jan 2016
Published date: 30 Jan 2016


