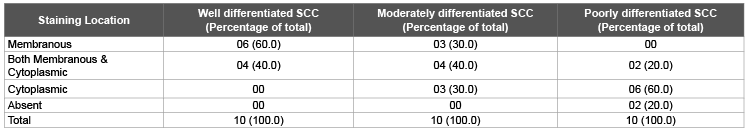
Table 1: Distribution of Staining Location of E-cadherin in different grades of Cervical squamous cell carcinoma
Pragati Agnihotri1 Kafil Akhtar*1 Shahid A Siddiqui2 Rana K Sherwani 1
1Department of Pathology, Jawaharlal Nehru Medical College, Aligarh Muslim University, Aligarh, India*Corresponding author: Dr. Kafil Akhtar, Associate Professor, Department of Pathology, Jawaharlal Nehru Medical College, Aligarh Muslim University, Aligarh.(U.P), India, E-mail: drkafilakhtar@gmail.com
Aims and Objectives: To evaluate whether expression of E-cadherin and Vimentin, and their specific pattern can predict invasiveness, and may be used as markers for early diagnoses and to correlate the therapy response and reveal prognostic importance of E-cadherin and Vimentin activity.
Material and Methods: Biopsies/ specimens of cervix uteri were evaluated for all premalignant lesions and invasive epithelial squamous lesions, by haematoxylin and eosin sections and by immunohistochemical expression of E-cadherin and vimentin. Patients follow up and therapy related changes were also studied.
Results: There were 111(37.0%) premalignant cases and 189 (63.0%) malignant cases in our study. Out of 10 cases, 9 cases (90.0%) of well differentiated, 8 cases (80.0%) of moderately differentiated while only 1 case (10.0%) of poorly differentiated carcinomas showed 4+staining for E-cadherin. One case (10.0%) of well differentiated carcinoma showed 1+(weak and focal) staining and one case (10.0%) each of moderately differentiated carcinoma showed 3+(strong and focal) and 4+(strong and diffuse) positivity for vimetin. One case (10.0%) of poorly differentiated carcinoma showed 2+(strong and focal) positivity, three cases each (30.0%) showed 3+(weak and diffuse) and 4+(strong and diffuse) staining for vimentin. Fischer exact test showed a ‘p value’ of <0.05, which was statistically significant. Conclusions: The immunohistochemical pattern of expression of E-cadherin and Vimentin could help to predict the prognosis and plan the management of the patient. Also, these biomolecules can be used as biomarkers for further research on the micro-invasion of the tumor for early diagnosis and survival of the patients.
Carcinoma cervix; Immunohistochemistry; E-cadherin; Vimentin
Cervical cancer is the fourth most common cancer in women, and seventh overall, with an estimated 528,000 new cases in 2012 [1]. There were estimated 266,000 deaths from cervical cancer worldwide in 2012, accounting for 7.5% of all female cancer deaths. Large majority (around 85%) of global burden occurs in the less developed regions, where it accounts for almost 12% of all the female cancers [1]. A vital step in determining newer modalities for reducing morbidity and mortality by cancer cervix, could be to find out interventions which intersect with the progression of cervical epithelial lesions into invasive cancer.
Major cause of mortality in carcinoma cervix patients is distant metastasis by lymphatic and vascular invasion, leading to treatment failure and recurrences [2]. Role of epithelial mesenchymal transition (EMT) in the process of metastasis has been widely accepted [3]. EMT is marked by loss of epithelial properties and acquisition of mesenchymal phenotype by the cell. During this process a more plastic ‘metastable phenotype’ is formed, which expresses both epithelial and mesenchymal properties [2]. This metastable cell has increased capability to migrate, increased resistance to apoptosis, increased chemoresistance and pleuripotent properties [3-5].
The phenomenon of EMT is marked by loss of epithelial marker E-cadherin and gain of mesenchymal marker Vimentin. Cadherins are transmembrane or membrane-associated glycoproteins involved in Ca2+ dependent cell-cell adhesion [6]. Loss or dysfunction of E-cadherin has been known to be associated with gain of invasive capacity and is correlated with high tumor grade and a poor prognosis [7]. The loss of E-cadherin-mediated cell adhesion is a hallmark of the transition from a normal epithelium to poorly-differentiated carcinoma [8].
The present study was undertaken to evaluate whether expression of E-cadherin and Vimentin, their specific pattern can predict invasiveness, and may be used as markers for early diagnoses and to correlate the therapy response and reveal prognostic importance of E-cadherin and Vimentin activity.
The present study was carried out on 300 cases of cervical lesions in the Department of Pathology, Jawaharlal Nehru Medical College, Aligarh. Biopsies/specimens of cervix uteri were evaluated for all premalignant lesions and invasive epithelial squamous lesions, using routine Hematoxylin and Eosin sections and by the presence of expression of E-cadherin and Vimentin, by immunohistochemistry, wherever possible.
Histopathology of paraffin embedded section of specimens was done using Hematoxylin and Eosin stain. Histologically proven cervical premalignant and malignant lesions were selected for the study. Subsequently, serial sections were performed, which were used for the immunohistochemical analysis, for which rabbit and mouse antihuman polyclonal antibodies were used. The working systems for the immunohistochemical reactions were represented by Novocastra ready to use mouse monoclonal antibodies for E cadherin and Vimentin. (DAB: 3,3’- diaminobenzidine, Dako). For the assessment of E-cadherin and Vimentin, immunohistochemically stained slides were examined for pattern of staining (nuclear, cytoplasmic or membrane), proportion and intensity of staining of the tumour cells. Staining intensity of E-cadherin was assessed in three patterns: (1) Strong- with uniform and strong staining in almost all the cells, (2) Weak and Homogeneoushomogeneous but weak staining than normal squamous epithelium, (3) Absent/Heterogeneous- Intensity of staining differed from cell to cell and negative cells without immunostaining were included [8]. Staining location of E-cadherin was graded as Membranous staining, Membranous and Cytoplasmic staining both, Cytoplasmic staining or Absent staining. On the basis of percentage of cells showing staining for E-cadherin, we introduced grades as follows: 0 - Negative membranous staining, 1+/- < 10% cells showing staining, 2+/- 10 to 20% cells showing staining, 3+/- >20 to <50% cells showing staining and 4+/- >50% cells showing staining. Vimentin staining was graded as absent or presents (> 5% cells). We also graded the staining intensity as: 0- Negative cytoplasmic positivity, 1+/- Weak and Focal cytoplasmic staining, 2+/- Strong and Focal cytoplasmic staining, 3+/- Weak and Diffuse cytoplasmic staining, 4+/- Strong and Diffuse cytoplasmic staining.
Fischer’s exact test was used for analysis of the immunorectivity results by E-cadherin and vimentin using SPSS ver. 15.0 (SPSS Inc., Chicago, IL, USA). All tests were two-sided and p-values less than 0.05 were considered statistically significant.
There were 111(37.0%) premalignant cases and 189 (63.0%) malignant cases in our study. Out of the 111 premalignant cases, 73 (65.8%) were low grade squamous intraepithelial lesion (LSIL) and 38 (34.2%) high grade squamous intraepithelial lesion (HSIL). Out of the total 189 malignant cases, 172 (91.0%) were squamous cell carcinoma, 8 (4.2%) small cell carcinoma, 5 (2.6%) adenocarcinoma and 4 cases (2.11%) were diagnosed as adeno-squamous carcinoma.
Immunohistochemical staining of malignant cervical squamous lesions by E-cadherin and Vimentin was studied in ten cases each of well, moderate and poorly differentiated squamous cell carcinoma and staining intensity, staining location and percentage of malignant cells showing immune-expression were studied and graded.
Nine cases (90.0%) of well differentiated squamous cell carcinoma showed strong staining intensity and 1 case (10.0%) showed weak and homogeneous staining intensity for E-cadherin. Strong staining intensity was present in 8 cases (80.0%) of moderately differentiated carcinomas, as compared to weak and homogeneous in 2 cases (20.0%). Out of 10, 1 case (10.0%) of poorly differentiated carcinoma showed strong and 7 cases (70.0%) showed weak and homogeneous staining while 2 cases (20.0%) were negative for staining. Fischer exact test showed a ‘p value’ of <0.05, which was statistically significant.
Six cases (60.0%) of well differentiated carcinoma showed membranous staining for E-cadherin as compared to 3 cases (30.0%) of moderately differentiated and none of poorly differentiated carcinoma. Both membranous and cytoplasmic staining was seen in 4 cases (40.0%) of well differentiated carcinoma, 4 cases (40.0%) of moderately differentiated carcinoma and 2 cases (20.0%) of poorly differentiated carcinoma. Three cases (30.0%) of moderately differentiated and 6 cases (60.0%) of poorly differentiated carcinoma showed cytoplasmic staining only. 2 cases (20.0%) of poorly differentiated carcinoma showed absence of staining. Fischer exact test showed a ‘p value’ of <0.05, which was statistically significant (Table 1).
Out of 10 cases, 9 cases (90.0%) of well differentiated, 8 cases (80.0%) of moderately differentiated while only 1 case (10.0%) of poorly differentiated carcinomas showed 4+ staining for E-cadherin (Figure 1). 3+staining was shown in 1 case (10.0%) of well differentiated, 2 cases (20.0%) of moderately differentiated and 2 cases (20.0%) of poorly differentiated carcinoma cases. 2 cases (20.0%) of poorly differentiated carcinoma cases showed 2+staining, while 1+in 3 cases (30.0%) and 0 in 2 cases (20.0%) poorly differentiated carcinoma cases. Fischer exact test showed a ‘p value’ of <0.05, which was statistically significant (Table 2).
Nine cases (90.0%) of well differentiated carcinoma, 8 cases (80.0%) moderately differentiated carcinoma and 3 cases (30.0%) of poorly differentiated carcinoma showed a negative staining for Vimentin. One case (10%) well differentiated carcinoma, 2 cases (20.0%) of moderately differentiated carcinoma and 7 cases (70.0%) of poorly differentiated carcinoma showed positivity for Vimentin staining. Fischer exact test showed a ‘p value’ of <0.05, which was statistically significant.
Negative staining for Vimentin was shown by 9 cases (90.0%) of well differentiated carcinoma, 8 cases (80.0%) of moderately differentiated carcinoma and 3 cases (30.0%) of poorly differentiated carcinoma. One case (10.0%) of well differentiated carcinoma showed 1+(weak and focal) staining. One case (10.0%) each of moderately differentiated carcinoma showed 3+(strong and focal) and 4+(strong and diffuse) positivity. One case (10.0%) of poorly differentiated carcinoma showed 2+(strong and focal) positivity, three cases (30.0%) showed 3+(weak and diffuse) and three cases (30.0%) showed 4+(strong and diffuse) staining (Figure 2) with a ‘p value’ of <0.05, which was statistically significant (Table 3).
Out of 18 cases strongly positive for E-cadherin, 15 cases (83.3%) were Vimentin negative while only 3 cases (16.7%) were positive for Vimentin staining. Six cases (60.0%), out of the 10 cases with weak and homogeneous staining for E-cadherin, were positive for Vimentin immunostaining and only 4 cases (40.0%) were Vimentin negative. All the cases (100.0%) with absence of E-cadherin positivity were Vimentin positive. Fischer exact test showed a ‘p value’ of <0.05, which was statistically significant (Table 4).
Frixen et al. [9] have stated that the ability of carcinomas to invade and to metastasize largely depends on the degree of epithelial differentiation within the tumours which was confirmed by examining various human cell lines derived from bladder, breast, lung, and pancreas carcinomas. They found that carcinoma cell lines with an epithelioid phenotype were noninvasive and expressed the epithelium-specific cell-cell adhesion molecule E-cadherin whereas carcinoma cell lines with a fibroblastoid phenotype were invasive and had lost E-cadherin expression.
Pfisterer et al. [10] have found a good correlation of E-cadherin expression with the state of differentiation in the cases of ovarian carcinoma. Carico et al. [11] have suggested that E-cadherin down-regulation might be associated with neoplastic transformation in laryngeal tissues. Perez et al. [12] have suggested that E-cadherin down-regulation, enhance the metastatic potential of undifferentiated endometrial carcinomas, leading to a poor prognosis. Tang et al. [13] have stated that reduced expression of E-cadherin significantly correlated with poor differentiation, advanced TNM stage, and lymph node metastasis of gastric cancers. Fan [14] has mentioned that low E-cadherin expression was associated with poor prognosis in oral squamous cell carcinoma.
In our study, E-cadherin showed a significant progressive loss of staining as the tumour differentiated from a well differentiated grade to a poorly differentiated grade. Nine cases (90%) of well differentiated carcinoma and 8 cases (80%) of moderately differentiated carcinoma showed strong membranous positivity, compared to only 1 case (10%) of poorly differentiated squamous cell carcinoma. Seven cases (70%) of poorly differentiated carcinoma showed homogeneous but weak staining for E-cadherin, compared to 2 cases (20%) of moderately differentiated carcinoma and 1 case (10%) well differentiated carcinoma. Kaur et al. [15] have studied E-cadherin expression in different histological grades of oral squamous cell carcinoma and reported strong expression in 90% well differentiated, 92.9% moderately differentiated and 15.4% poorly differentiated cases as compared to weak and heterogeneous staining showed by 10%, 7.1% and 69.2% cases respectively. Loss of E-cadherin expression was documented in 15.4% of poorly differentiated carcinoma, 0% of well and moderately differentiated carcinoma each. Mehendiratta et al. [16] have reported absence of staining in 0%, 10%, 30% of well, moderate and poorly differentiated oral squamous cell carcinoma, respectively.

Table 1: Distribution of Staining Location of E-cadherin in different grades of Cervical squamous cell carcinoma
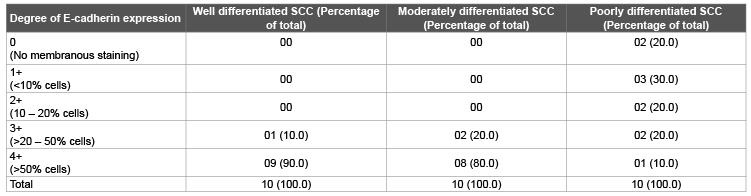
Table 2: Distribution of E-cadherin positivity as percentage of total malignant cells
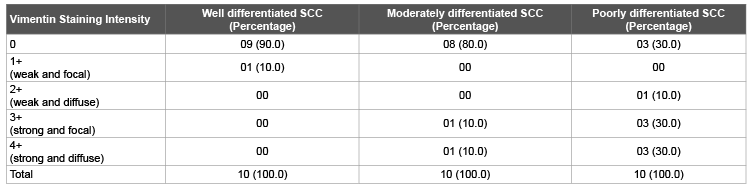
Table 3: Distribution of Vimentin staining on basis of Staining Intensity in malignant lesions
Our study showed, cytoplasmic staining in 3 cases (30.0%) of moderately differentiated and 6 cases (60.0%) of poorly differentiated carcinomas. Quite similarly Kaur et al. [15] have found cytoplasmic staining in 28.6% moderately differentiated and 61.5% poorly differentiated oral SCC cases. Myong et al. [7] have documented a tendency for increase in cytoplasmic E-cadherin immunoreactivity and loss in membranous immunoreactivity as lesion progressed from CIS to microinvasive and invasive SCC, respectively. Cytoplasmic staining was reported as 51%, 71%, and 95%, respectively and membranous was recorded as 49%, 23%, and 0%, respectively.
In our study, Grade 4+staining for E-cadherin was seen in 90% of well differentiated carcinoma cases, 80% of moderately differentiated carcinomas and only 10% of poorly differentiated carcinoma cases. We noted a trend of decrease in percentage of cells stained positive with degree of differentiation of tumours. Tang B et al. [13] have reported that reduced expression of E-cadherin is significantly correlated with poor differentiation, advanced TNM stage, and lymph node metastasis of gastric cancers.
In our study, 1 case (25%) with strong E-cadherin staining and 1 case (25%) with weak & homogeneous staining in primary tumour showed lymph node metastasis, as compared to 2 cases (50%) of primary tumours with heterogeneous staining or lack of staining. Mehendiratta et al. [16] have documented 21 cases that were positive for lymph node metastasis, out of these 10 cases (47%) showed a moderate expression and 4 cases (19%) showed absent or mild expression for E-cadherin.
In present study, progressive gain of Vimentin was seen with increasing grade of the tumour. None of the well differentiated tumours showed vimentin positivity, while 20% moderately differentiated and 70% poorly differentiated tumours were positive for Vimentin expression. Only 20% moderately differentiated tumours showed diffuse Vimentin positivity (Grade 3+and 4+) as compared to 60% poorly differentiated tumours. Liu et al. [17] have stated that high expression of Vimentin was observed in 53% tumours from patients who eventually developed a recurrent tumour and was associated with recurrence and death. Domagala et al. [18] have found Vimentin expression to be a strong indicator of poor prognosis in node-negative ductal NOS breast carcinomas.
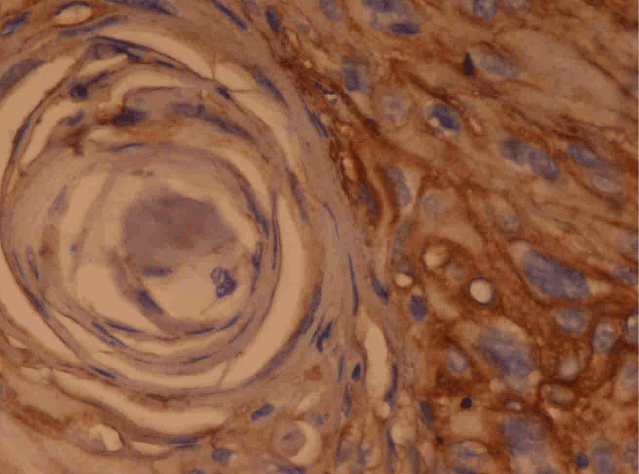
Figure 1: Well differentiated Squamous Cell Carcinoma: Photomicrograph shows Strong, Membranous, Grade 4+staining in the tumour cells. (IHC: E-cadherin × 40X)
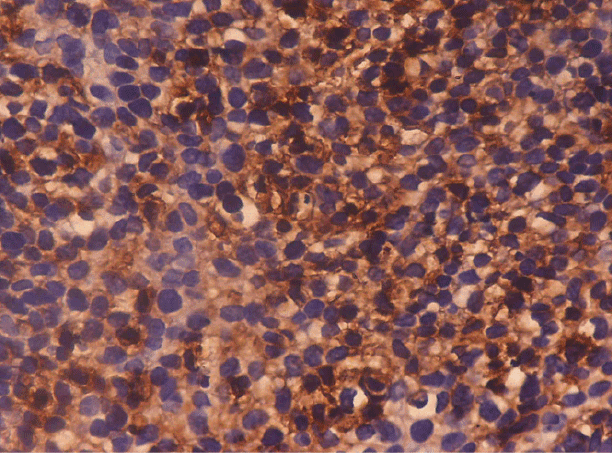
Figure 2: Poorly Differentiated Squamous Cell Carcinoma: Photomicrograph shows Grade 4+ cytoplasmic staining in the tumour nest. (IHC Vimentin × 40X)
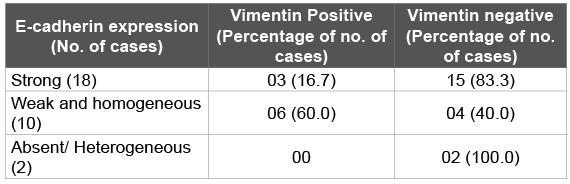
Table 4: Inverse correlation between E-cadherin and Vimentin expression in cervical squamous cell carcinoma cervix
Inverse relationship between E-cadherin and Vimentin expression was seen (p<0.05) in the 30 cases of carcinoma cervix studied by us. Thompson et al. [19] have also concluded that human breast cancer progression results first in the loss of E-cadherin, and subsequently in Vimentin acquisition, the latter being associated with increased metastatic potential through enhanced invasiveness. Myong et al. [7] have documented that there was an inverse relationship between E-cadherin and Vimentin expressions. In their study, 45% cases documented a strong Vimentin expression in cases with reduced E-cadherin expression, as compared to only 10% cases with Vimentin expression in cases with strong membranous E-cadherin positivity.
Liu et al. [17] have studied oral squamous cell carcinoma and documented high expression of Vimentin in 23 of 43 (53%) tumours from patients who eventually developed a recurrent tumour and was associated with recurrence and death (P<0.001 and <0.001, respectively). The decreased expression of E-cadherin was observed in 36 of 43 (84%) tumours from patients who eventually developed a recurrent tumour and was also associated with recurrence and death (P<0.001 and <0.001, respectively). The combination of the upregulation of Vimentin and aberrant expression of E-cadherin/β-catenin complexes at the tumour invasive front may provide a useful prognostic marker in oral squamous cell carcinoma.
In our study, down-regulation of E-cadherin and up-regulation of Vimentin was positively related to histological differentiation, lymph node metastasis. These findings provide significant evidence in favour of the role of epithelial mesenchymal transition (EMT) in progression of cancer cervix and in prediction of invasiveness of various intraepithelial and epithelial lesions.
E-cadherin showed a significant loss of staining intensity, decrease in number of cell showing staining and increase in cytoplasmic staining along with decrease in membranous staining and Vimentin showed a significant gain in positivity and staining intensity as the tumour differentiated from well to poor. The immunohistochemical pattern of expression of E-cadherin and Vimentin could help to predict the prognosis and plan the management of the patient.
Download Provisional PDF Here
Article Type: Research Article
Citation: Agnihotri P, Akhtar K, Siddiqui SA, Sherwani RK (2015) Cervical Cancer Metastasis: Significance of E-Cadherin and Vimentin. Clin Res Open Access 1(2): doi http://dx.doi.org/10.16966/2469-6714.107
Copyright: © 2015 Agnihotri P, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access