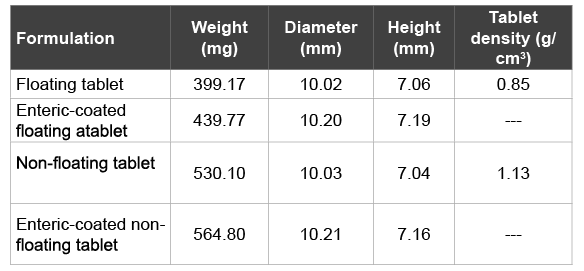
Table 1: Properties of the convex-shaped round tablets
Veronika A. Eberle1 Massimiliano Donzelli2 Rainer Alles1 Maxim Puchkov1 Jörg Huwyler1,*
1Department of Pharmaceutical Sciences, Division of Pharmaceutical Technology, University of Basel, Klingelbergstrasse 50, 4056 Basel, Switzerland*Corresponding author: Prof. Dr. Jörg Huwyler, Division of Pharmaceutical Technology, Department of Pharmaceutical Sciences, University of Basel, Klingelbergstr. 50, 4056 Basel, Switzerland, Tel: + 41 (0)61 267 15 13; Fax: + 41 (0)61 267 15 16; E-mail: joerg.huwyler@unibas.ch
Floating dosage forms are supposed to exhibit an enhanced gastric residence time. Their development is challenging as the prediction of the retention potential in humans based on in vitro studies and animal models is difficult.
A strategy to determine the stomach residence time of a floating dosage form with an inherently low density in human without using imaging techniques was explored in a self-experiment.
Floating tablets and non-floating controls were prepared containing caffeine as a model drug. The compacts had a pH-dependent entericcoating to assess their stomach residence time. Since caffeine is rapidly absorbed in the gastrointestinal tract, the prolonged gastric retention of tablets can be demonstrated by a delayed systemic exposure. Caffeine and paraxanthine were determined in capillary blood by liquid chromatography coupled to tandem mass spectrometry.
An increase in caffeine and paraxanthine blood levels was observed in human volunteers after 90 to 180 min for the non-floating controls. For the floating tablets, no elevated blood concentrations were found in two out of three participants during 8 h of sample collection.
The results demonstrate the technical feasibility of the proposed clinical study protocol. Follow-up clinical trials will be needed to confirm the preliminary data on stomach residence time of our floating dosage form.
Gastroretentive dosage form; Floating tablet; In vivo gastric residence time; Self-experiment; LC-MS/MS
GRDDS - Gastroretentive drug delivery systems; FDDS - Floating drug delivery systems; FCC - Functionalized calcium carbonate; LC-MS/ MS - Liquid chromatography coupled to tandem mass spectrometry
Gastroretentive drug delivery systems (GRDDS) offer the possibility to prolong and control the residence time of drugs in the stomach and the upper part of the small intestine [1]. The reason and need for a gastroretentive delivery approach is in most cases dictated by physiological necessity [2]. The passage time of conventional oral pharmaceutical dosage forms through the stomach is relatively short; thus, GRDDS provide a technology to overcome this limitation. Several drug substances, e.g. drugs acting locally or being absorbed in the stomach region might benefit from enhanced gastric retention times [3]. Floatation has been proposed as a mechanism to avoid unpredictable and premature gastric emptying of pharmaceutical dosage forms [4]. Due to the low density of the drug delivery systems, they float on stomach contents and are retained while releasing the drug substance.
During the last years, the development of floating drug delivery systems (FDDS) has gained importance. However, there are only few examples of clinical trials or marketed GRDDS. One explanation of this shortcoming is the fact that it is very difficult to extrapolate from in vitro studies or from animal experiments to human. Thus, the phenomenon of flotation has to be explored in human to account for the unique anatomical and physiological situation in humans. Nevertheless, even complex clinical trial protocols often fail to show significantly enhanced gastric residence times of FDDS in comparison to non-floating references. A number of shortcomings are associated with the clinical evaluation of floating dosage forms: the gastric retention time is often determined in the fed state; although, high-caloric food is known to delay the gastric emptying rate [5]. Additionally, numerous researchers select non-appropriate nonfloating control dosage forms, e.g. immediate release formulations, in order to prove prolonged gastric residence times. In some clinical studies, only pharmacokinetic data are recorded to provide an indirect estimate of the retention time of a dosage form in the stomach.
A FDDS, which is based on the use of the porous carrier functionalized calcium carbonate (FCC), was developed and in vitro flotation in a custombuilt stomach model was demonstrated. Tablet dissolution and buoyancy behavior were simulated in silico and provided insight into drug release and floating mechanism [6]. But, the extrapolation from these model systems to the in vivo situation proofed to be a challenge. Therefore, it was decided to evaluate a strategy to test the gastric retention potential in human of FCC-based tablets featuring an inherently low density. The aim of the present project was to design and evaluate a method in human, which can be used to assess and compare the in vivo gastric residence time of floating and non-floating tablets. The compacts contained caffeine as a model drug substance and were coated with a pH-dependent enteric-coating. Upon retention of a tablet in the acidic environment of the stomach, the marker caffeine is thus retained within the tablet. Using minimal amounts of capillary blood, the systemic exposure of caffeine and its metabolite paraxanthine were determined using liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS).
Highly porous functionalized calcium carbonate (FCC VP-DP141 S04, Omya, Oftringen, Switzerland) [7] was used as matrix for the preparation of tablets with an inherently low density. Two gelation-layer forming polymers, water-soluble polyethylene oxide (Polyox™ WSR 301, The Dow Chemical Company, Midland, Michigan) and hydroxypropyl methylcellulose (Methocel® K100 Premium LV, Sandoz Pharma AG, Basel, Switzerland) were selected to slow down penetration of liquid into the tablet and retard release of the model drug substance caffeine (Coffeinum WSF, Böhringer-Ingelheim, Ingelheim, Germany). Citric acid (Acid citricummonohydr. pulvis, Hänseler AG, Herisau, Switzerland) was chosen as effervescent excipient. The floating formulation was prepared by wet-granulation with ethanol 96% (SchweizerhallChemie AG, Flawil, Switzerland). The enteric-coating suspension consisted of the following materials: Kollicoat MAE 30 DP (BASF, Germany), propylene glycol (Hänseler AG, Switzerland), talc (Hänseler AG, Switzerland), and titanium dioxide (Hänseler AG, Switzerland).
Preparation of floating and non-floating tablets: Floating tablet formulation was prepared as described
previously [6]. For in vivo investigation of gastric retention times, floating and non-floating tablets using a single punch eccentric press (Styl’One, Medelpharm, France) were manufactured. The convex-shaped tablets had a curvature radius of 12 mm and a tablet radius of 5mm. The cap height was 1.1 mm. The caffeine containing formulation had the same composition for both the floating and the non-floating tablets. Tablet density was adjusted by using different compaction forces. Since nonfloating tablets had a reduced tablet height, an additional layer of 130 mg pure FCC was added to obtain identically sized floating and non-floating control tablets. Thus, an impact of tablet size on gastric retention could be excluded. The characteristics of the prepared compacts are summarized in Table 1.
After compaction, the floating and non-floating tablets were coated with an enteric-coating in a drum coater (Lab-Coater GC-300, Glatt, Switzerland).
Evaluation of in vivo gastric retention in humans: Prior to the in vivo evaluation, drug release and flotation behavior of the enteric-coated tablets were studied in vitro using a custom-built stomach model [6]. Nonfloating tablets were expected to remain in the gastric region for about 2 to 3 h [8]. Within this time, the enteric-coating was supposed to remain intact; thus, no caffeine and paraxanthine should be observable in the blood samples of the volunteers. After being emptied from the stomach, the enteric-coating of the compacts should dissolve due to a pH change in the intestine, and caffeine as well as paraxanthine plasma concentrations increase. In the case of the gastric-coated floating dosage forms, no caffeine and paraxanthine should be present in the blood samples of the volunteers during 8 h of sample collection.

Table 1: Properties of the convex-shaped round tablets
Study design: The exploratory experiment in human volunteers (n=3) using the marker caffeine was brought to the attention of the local ethics committee (EKNZ; reference number UBE-15/17) and was considered by the committee to be compliant with Swiss legislation. All participants gave informed consent prior to the study.
The study started with a caffeine washout period of 3 days; the participants were not allowed to consume any caffeine-containing food. In order to evaluate the in vivo gastric retention times, tablets were administered at 08:00 a.m. with 300 ml of water after an overnight fasting. Every half hour, 300 ml of liquid was provided. A standardized, lowcaloric liquid meal was given 4 h post-administration. Blood samples of 10 µl were collected via capillary puncture of the fingertip at the indicated times. Collected blood samples were diluted with 200 µl methanol containing 50 ng/ml of caffeine-d9 as the internal standard and stored at -20 °C for further analysis.
Determination of caffeine and its metabolite paraxanthine in human plasma: Blood concentrations of caffeine and paraxanthine were determined in collected blood samples using LC-MS/MS. The analytes were extracted by protein precipitation using methanol (see above). Chromatographic separation of sample supernatants was done on a Shimadzu HPLC system consisting of an HTS PAL autosampler (CTC Analytics AG, Switzerland), two Shimadzu LC-20 AD pumps (Shimadzu AG, Switzerland) controlled by a Shimadzu CBM-20A unit, and a Shimadzu CTO-20AD column oven. An Atlantis T3 column (2.1 x 50 mm, Waters AG, Switzerland) was used to separate the analytes. Eluent A (0.1% formic acid in water) and eluent B (0.1% formic acid in methanol) were delivered at a constant flow of 0.8 ml/min. The following gradient was applied: 100% A from 0 to 0.5 min, 50 – 98% B from 0.5 to 2 min, 98% B from 2 to 2.5 min, 100% A from 2.5 to 2.8 min. Total run time was 2.8 min. A thermo stated column oven was set to 60 °C. The injection volume was 10 μl. The flow was directed to waste for the first and last 0.6 min, to minimize contamination of the MS source. Mass spectrometric detection was performed using a triple quadrupole mass spectrometer (API4000, Applied Biosystems, Switzerland) operating in electrospray-ionization positive-ion mode. Samples were quantified using peak area ratios and caffeine-d9 as the internal standard. The assays were linear in the concentration ranges of 25 – 1000 ng/ml for caffeine and paraxanthine.
Statistical analysis: A one-sided Grubb’s test for outliers with a 90% confidence interval was used in order to detect outliers in the in vivo caffeine plasma concentration profiles of floating tablets [9].
Figures 1a-1c displays the caffeine and paraxanthine blood concentrations measured in human volunteers (n=3) after administration of the prepared non-floating enteric-coated tablets. Participants 1, 2, and 3 showed no elevated caffeine and paraxanthine plasma concentrations up to 90, 126, and 175 min, respectively. Thus, the enteric-coating of the compacts remained intact within this time period and no caffeine was released from the tablets. We assume that the control tablets were located in the stomach of the participants during this time.Afterwards,a caffeine release from the tablets was observed, resulting in an increase in caffeine and paraxanthine levels. It can be concluded that the tablets passed through the pylorus into the small intestine. Due to a change in pH in the intestinal environment, the enteric-coating started dissolving and caffeine was released. Consequently, blood concentrations of caffeine and its metabolite paraxanthine increased. For the non-floating tablets, the time points at which caffeine and paraxanthine blood concentrations increased after administration were comparable for all three study participants. The results were in good agreement with another study performed by Podczeck et al., where the mean gastric residence time was determined to be 91 min for 10 mm tablets administered with a dextrose drink. The gastric emptying of food and its influence on the gastric emptying of tablets of different dimensions was measured by γ-scintigraphy and electrical impedance tomography [10].

Figures 1: In vivo caffeine (□) and paraxanthine (■) concentrations in the plasma of three human volunteers after administration of the non-floating enteric-coated control tablet. Each data point represents one blood sample.
In contrast, in two out of three participants no increase in caffeine and paraxanthine concentrations was measured during 8 h blood sample collection for the floating tablets (Figures 2a and 2b). Elevated caffeine plasma concentrations were observed at isolated time points. However, high caffeine levels were not accompanied by high paraxanthine levels. No systemic exposure of caffeine and its metabolite paraxanthine could be detected; hence, indicating that the floating dosage forms remained in the human stomach.
In the case of one study participant, plasma profiles after administration of the floating enteric-coated tablet were similar to the ones of the nonfloating tablet; an increase in caffeine and paraxanthine concentrations was seen after approximately 90 min. In this situation, it was not possible to determine the reason for failure of the experiment. It might be that either the enteric coating of the tablet was defective or that the tablet was emptied prematurely from the stomach.
The pilot experiment did reveal shortcomings of the chosen study design. Blood sample collection during 8 h was found to be not sufficient in order to detect the increase in caffeine and paraxanthine plasma concentrations caused by gastric emptying of the FDDS. First, blood sampling time should be extended to at least 24 h to cover longer than expected gastric retention times of the tablets. Second, increased caffeine levels at isolated time points were obtained in two volunteers. They were identified as outliers based on statistical analysis and the absence of a concomitant occurrence of the caffeine metabolite paraxanthine. We concluded that these outliers were due to a contamination of samples by caffeine. In addition, analytical methods have to be used which cover both caffeine and its metabolite paraxanthine to exclude blood sample contaminations. It might be advisable to validate in a second step the position of the tablets in the stomach by e.g. γ-scintigraphy and not to rely exclusively on pharmacokinetic analysis of plasma profiles of a marker substance.
The volunteers in this study were provided with low-caloric and liquid food in order to exclude the effects of high-caloric meals, which delay the gastric emptying process and thus influence the gastric retention of pharmaceutical dosage forms. The amount of liquid and food consumed during the experiment should therefore be strictly controlled. The volunteers had to maintain an upright position during the duration of the experiment; however, they were allowed to move and to do light exercise. Our preliminary results seem to suggest that body motion was not a critical factor.
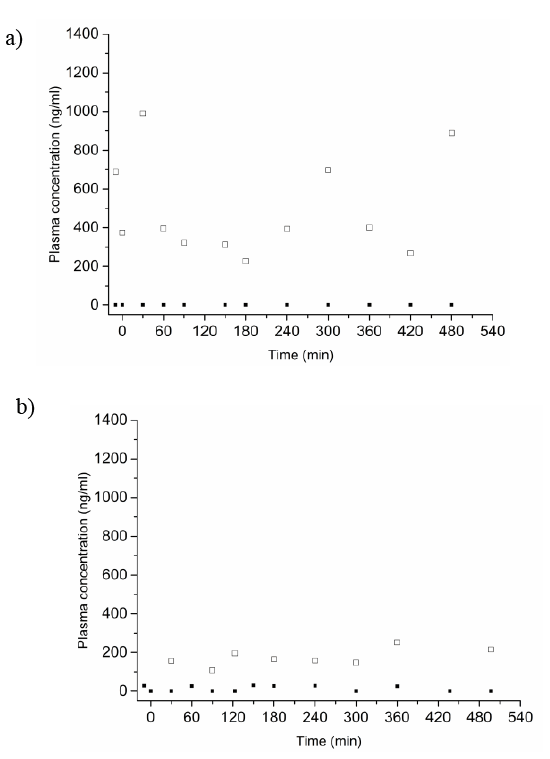
Figures 2: In vivo caffeine (□) and paraxanthine (■) concentrations in the plasma of two human volunteers after administration of the floating enteric-coated tablet. Each data point represents one blood sample. Caffeine outliers identified by a one-sided Grubb’s test for outliers with a 90% confidence interval are not shown on the graph.
Coating of tablets with a pH-dependent enteric-coating seems to be an interesting strategy to compare the retention times in the human stomach of floating tablets with non-floating reference systems. Non-floating controls can be designed to differ only by their density from the test tablets. They are superior to immediate-release formulations, which are often used as controls. The use of the marker component caffeine seems to be an alternative to more expensive imaging techniques.
It was the aim of the present pilot study to demonstrate the technical feasibility of a clinical study protocol. Obtained preliminary results demonstrate that in vivo characterization in human of a floating dosage form is possible. Floating and non-floating control tablets can be compared without applying complex imaging technologies.
The performed exploratory study is encouraging and will therefore serve as a basis for the preparation of clinical phase I studies to evaluate the properties of FCC-based floating dosage forms. In addition, the pilot experiment will help to define study conditions and parameters. The experience gained from this exploratory study might convince other researchers that in vivo performance of FDDS can potentially be explored using a relatively simple clinical study design. We hope that our preliminary findings might therefore promote the use of this innovative drug delivery strategy. Follow-up clinical trials using larger cohorts of volunteers will be needed to obtain statistically significant data on stomach residence time of our floating dosage form.
Rainer Alles, Dr. Maxim Puchkov, and Prof. Dr. Jörg Huwyler have contributed equally to the present work. Financial support for the Ph.D. thesis of Veronika Eberle was kindly provided by OmyaInternational AG (Oftringen, Switzerland).Many thanks to Dr. TanjaStirnimann for her support during the study.
Download Provisional PDF Here
Aritcle Type: Research Article
Citation: Eberle VA, Donzelli M, Alles R, Puchkov A, Huwyler J (2015) In Vivo Evaluation of a Gastroretentive Drug Delivery System Based on Enteric-Coated Floating Tablets. Clin Res Open Access 1(2): doi: http://dx.doi.org/10.16966/2469-6714.105
Copyright: © 2015 Huwyler J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access