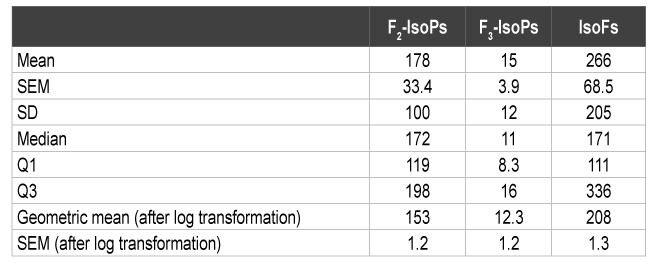
Table 1: Plasma Concentrations of Isoprostanes and Isofurans (pg/ml)
Stan Kubow1* Matthew Sorenson2 Dalal Alkazemi3 Jackson Roberts L II4 Katherine Naselli Adamski5 Leonard A Jason5
1School of Dietetics and Human Nutrition, McGill University, Montreal, QC, Canada*Corresponding author: Stan Kubow, School of Dietetics and Human Nutrition, McGill University, 21,111 Lakeshore, Ste-Anne-de-Bellevue, Quebec H9X3V9, Canada, Tel: 514-398-7754; Fax: 514-398-7739; E-mail: stan.kubow@mcgill.ca
Plasma levels of F2 -isoprostanes (F2 -IsoPs), a whole body oxidative stress marker, have been noted to be elevated in populations with chronic fatigue syndrome (CFS). We hypothesized that the oxidative stress biomarkers of F2 -isoprostanes (F2 -IsoPs), isofurans (IsoFs) and F3 -isoprostanes (F3 -IsoPs) are related to hypothalamic-pituitary-adrenal (HPA) axis dysregulation in chronic fatigue syndrome (CFS) subjects assessed by plasma dehydroepiandrosterone (DHEA) and cortisol and gene expression of sirtulin-1 (SIRT1). Plasma samples were obtained from nine CFS individuals aged 65 years or older. The mean levels of plasma F2 -IsoPs (M=178 pg/ml, SD=100) and IsoFs (M=266 pg/ml, SD=205) were several-fold higher than previously reported in healthy subjects. F2 -IsoPs:F3 -IsoPs and SIRT1 were negatively associated (r=– .674, p<.05) whereas F3 -IsoPs were inversely correlated with DHEA (r=– .586, p<.05) and positively associated with SIRT1 (r=.733, p<.05). After correction for age and sex, the F2 -IsoPs:IsoFs ratio was positively correlated to DHEA:cortisol (r=.758, p<.05).The findings indicate that plasma IsoFs and F2 -IsoPs are elevated in older subjects with CFS and that isoprostane and isofuran ratios are associated with HPA dysregulation.
Chronic fatigue syndrome; Isoprostanes; Isofurans; Cortisol; Hypothalamic-pituitary-adrenal axis; Mass spectrometry
Dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis is associated with an altered physiological response to stress with chronic fatigue syndrome (CFS) [1]. Subjects with CFS have lower cortisol secretion [2], glucocorticoid resistance [3] and a disruption of the expected diurnal cortisol pattern [4]. In our previous study involving elderly CFS subjects, decreased genetic expression of the glucocorticoid receptor (NR3C1) was related to low levels of plasma cortisol [5]. Cortisol is influenced by dehydroepiandrosterone (DHEA), a hormone that has been implicated in the pathogenic process of fatigue [6]. Our study showed that gene expression of sirtulin-1 (SIRT1) was strongly negatively correlated with plasma DHEA [5]. In the presence of diseases affecting the immune response, such as CFS, the homeostatic ability to counter the effects of oxidative stress is often decreased and can also be measured via the ferric reducing ability of plasma (FRAP) [5]. We suggested that disruption to HPA axis function could be partly mediated via oxidative stress as a decrease in the FRAP antioxidant capacity values was noted [5] but this study left unanswered questions regarding the relationship of indicators of HPA function with direct biomarkers of lipid peroxidation.
F2 -Isoprostanes (F2 -IsoPs) are stable products derived from noncyclooxygenase free radical-catalyzed peroxidation of arachidonic acid after its esterification to membrane phospholipids and subsequent release via phospholipase activity [7]. Measurement of F2 -IsoPs via gas chromatography-negative ion chemical ionization-mass spectrometry (GC-NICI-MS) is widely used to explore the role of oxidative stress in many clinical studies. Higher F2 -IsoPs levels have been observed in adults [8] and children [9] with CFS as compared to age and sex-matched healthy controls; however, the concurrent measurement of F2 -IsoPs with isofurans (IsoFs) and F3 -isoprostanes (F3 -IsoPs) could provide a more accurate assessment of the relationship between oxidative stress and HPA dysregulation. In that regard, formation of F2 -IsoPs is impaired at elevated oxygen tensions whereas in circumstances of decreased mitochondrial oxygen consumption that causes elevated intracellular oxygen tension, a preferential production of isofurans (IsoFs) occurs [10]. Thus, the ratio of F2 -IsoPs versus IsoFs is determined by low and high tissue oxygen tension, respectively. F3 -isoprostanes (F3 -IsoPs) are derived from another polyunsaturated fatty acid, eicosapentaenoic acid, and have been recently indicated as another chemically stable isoprostane marker of lipid peroxidation generated in vivo [11]. Moreover, the presence of different isoprostane species and IsoFs in relationship to CFS has not been previously characterized. The objectives of the present study were to determine plasma levels of F2 -IsoPs, F3 -IsoPs and IsoFs and characterize their relationship to plasma cortisol, DHEA and FRAP values and gene expression of SIRT1. We hypothesized that our subjects would exhibit elevated levels of F2 -IsoPs, F3 -IsoPs and IsoFs and that the ratio of DHEA to cortisol would be associated with these indicators of oxidative stress. We also theorized that the above oxidative stress markers would negatively correlate with FRAP indicating the protective role of antioxidant status.
We examined data from 6 females and 3 males with an average age of 69 (range 65-79) who were diagnosed with CFS using the Fukuda criteria [12]. Plasma was prepared from blood samples obtained from the Whittemore Peterson Institute for Neuro-immune Disease and stored at -80°C until time of analysis. Following extraction and derivatization of plasma, F2 -IsoPs, F3 -IsoPs and IsoFs were quantified by GC-NICI-MS in a single assay as previously described [13]. Briefly, lipids were extracted from tissue by the Folch method (CHCl3 /methanol 2:1, v/v), containing 0.005% butylated hydroxytoluene. The lipids were evaporated to dryness and hydrolyzed with KOH (15%) to release F2 -IsoPs, F3 -IsoPs and IsoFs. The F2 -IsoPs, F3 -IsoPs and IsoFs were then extracted using a C18 SepPak column, converted to pentafluorobenzyl esters, purified by thin layer, derivatized to trimethylsislyl ether derivatives, and quantified by GC-NICI-MS using [2H4 ] 15-F2 t-isoprostane as an internal standard. An Agilent 5973 Mass Spectrometer coupled to an Agilent 6890N GC using a 15 mDB 1701 GC column with an inlet temperature of 260°C. The helium carrier gas flow rate was 2 ml/min. For sample injection, the GC oven was programmed to run from 190 to 300°C at 20°C/min for 9 mins. Selective ion GC/NICI/MS monitoring was 569 m/z for F2 -IsoPs, 567 m/z for F3 -IsoPs, 585 m/z for IsoFs and 573 m/z for the internal standard [2H4 ] 15-F2 t-isoprostane. Values are expressed in picograms per milliliter of plasma. The precision of the assay is ± 6% and the accuracy is 96%.
All variables were treated on a continuous scale. Variables displaying undesirably high skew or kurtosis were transformed using natural logarithms to allow for assessment via parametric tests. Pearson’s correlation analysis was used to assess the relationship between plasma levels of oxidative stress measures and other study parameters. Partial correlations were performed to account for the effects of gender and age. All p values are for two-tailed tests, and a significance level of .05 was considered acceptable. Analyses were conducted in SPSS (Version 19, SPSS, Inc., Chicago, IL).
The current study is based on the same samples used in our previous work describing in more detail the relationship between HPA axis dysregulation, lower total antioxidant power and decreased expression of the gene encoding for the glucocorticoid receptor [5]. The plasma F2 - IsoPs and IsoFs values obtained from the CFS subjects in the present study (Table 1) were clearly greater than the normative steady state ranges of healthy subjects established previously for these biomarkers. All subjects had plasma F2 -IsoPs levels (M=178 pg/ml) well above the upper values previously reported for well-functioning older adults (median=54.3 pg/ ml - interquartile range 41.6 to 72.8 pg/ml) [14] and in a similar range to children with CFS (M=252 pg/ml, SD=44) [9] but lower than middleaged adult CFS subjects (M=406 pg/ml, SD=192) [8]. A previous report involving acutely ill geriatric patients showed plasma F2 -IsoPs median levels of 30 pg/ml with a range of 10-130 pg/ml based on the figure presented in that study [15]. The F3 -IsoPs were detectable (M=15 pg/ ml) in contrast to undetectable F3 -IsoPs levels in obese-hyperlipidemic or hypertensive type 2 diabetic men [16] but were similar to levels in healthy subjects in another study using GC-NICI-MS [17]. The plasma IsoFs values were about 9-10 fold higher (M=266 pg/ml) as compared overweight Inuit adults (M=20.8 pg/ml, SD=2) [18] and to healthy adults (M=37.7 pg/ml, SD=5.7) [19]. These findings further support the concept that CFS is associated with oxidative stress as noted previously [8,9]. In agreement with the above F2 -IsoPs and IsoFs results, the mean FRAP value of these subjects reported in our previous work [5] (M=227.3 µmol/l, SD=173) was substantially lower than previously reported values for older individuals. In addition, plasma concentrations of IsoFs and F3 -IsoPs showed a clear tendency to be negatively associated with FRAP values after adjustment for possible confounders of age and sex (Table 2). Taken together, the findings presented herein support the concept that CFS is associated with an imbalanced antioxidant response to oxidative stress. The above findings are also in concordance with previous CFS studies showing reduced plasma antioxidant levels [9] and raised levels of oxidized low-density lipoproteins [8], which have been suggested to be secondary to exacerbated inflammatory processes in CFS [8].

Table 1: Plasma Concentrations of Isoprostanes and Isofurans (pg/ml)
An imbalanced ratio of high DHEA and low cortisol secretion has been indicated in situations of chronic stress [20]. Lower cortisol levels are a frequent finding in studies of those with CFS [21], which points toward the dysregulation of the HPA axis. Likewise, our previous analysis of the samples measured in the present study showed considerably lower plasma cortisol levels (M=64.36 ng/ml, SD=28.7) that reported values for healthy controls and markedly higher DHEA levels (M=21.68 ng/ml, SD=12.5) than observed in similarly aged healthy elders [5]. In addition to their role as biomarkers of oxidative stress, F2 -IsoPs can exert potent pathophysiological actions [22] and so F2 -IsoPs could be a direct physiological mediator of HPA dysfunction in CFS. F2 -IsoPs have been indicated to be a biomarker of neuropathologies such as multiple sclerosis and Alzheimer’s disease [23]; however, IsoFs are preferentially elevated relative to F2 -IsoPs in other neurodegenerative conditions such as Parkinson’s disease [24]. Previous studies have related chronic oxidative stress exposure to damage to the hippocampus and the HPA axis as oxidative stress can lead to neural apoptosis and loss [25]. In that regard, it is noteworthy that the ratio of F2 -IsoPs:IsoFs showed a significant positive association with the DHEA:cortisol ratio after adjustment for the confounding factors of age and sex (Table 2). Also, there was a tendency of negative association of F2 -IsoPs:IsoFs with SIRT1 expression (p<0.1) that is strongly negatively correlated with DHEA (Table 2). In contrast, F3 -IsoPs showed a negative relationship with plasma DHEA and a positive correlation with SIRT1 although they were not significant after adjustment for age and sex (Table 2). These latter observations could be related to the competing biological effects of F3 -IsoPs with F2 -IsoPs as F3 -IsoPs have been suggested to exert anti-inflammatory effects that counteract proinflammatory F2 -IsoPs [11].
The exceptionally high levels of IsoFs could be of relevance in view of elevated IsoFs levels noted in diseases with mitochondrial dysfunction such as Parkinson’s disease [24]. Mitochondrial failure has been suggested for CFS as an outcome of excitotoxic effects secondary to centrally mediated kindling [26]. The high IsoFs levels support the concept of mitochondriopathy in CFS as inadequate mitochondrial activity results in elevated intracellular oxygen tension leading to preferential production of IsoFs [10]. Thus, the markedly high plasma IsoFs concentrations noted in the present CFS samples compared with relatively lower concentrations of F2 -IsoPs, support the concept of mitochondriopathy in CFS. In contrast to F2 -IsoPs, the bioactive effects of IsoFs are not well researched as only a few studies have measured IsoFs as markers of oxidative stress. Hence, the pathophysiological consequence of the modification in free radical oxidation towards IsoFs from F2 -IsoPs is unclear. This data, however, provides suggestion that plasma IsoFs have the potential to be utilized as a valuable biomarker of CFS in the future.
Although the sample size is small in the present study, it is in the range of other CFS preliminary uncontrolled studies that have evaluated subject characteristics [27,28]. The extrapolation of the findings from the present study, however, is limited by the small sample size and the partial examination of a number of variables influencing HPA axis function. Larger studies in the CFS population are needed to validate or refute our findings. As we were unable to include normative control samples, previous research literature values were used to indicate normal concentrations of hormonal values, antioxidant measures and isoprostane concentrations. Since this is a cross-sectional study, we cannot conclude a causal relationship between isoprostane values and HPA axis dysregulation. Possible confounding factors such as antioxidant supplement use, smoking and drinking status, prescription drug use; chronic disease status and other unmeasured confounders could not be adjusted for. As the samples were limited to older adults, the results may not be generalizable to the overall adult CFS population or to younger CFS age groups.
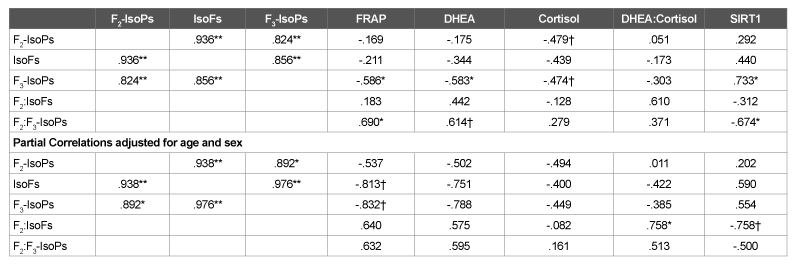
Table 2: Pearson’s Correlations between Plasma Biomarkers of Oxidative Stress and Metabolic Biomarkers
In conclusion, this is the first study to show a relationship between F2 -IsoPs, F3 -IsoPs and IsoFs with indices of HPA dysregulation, which provides preliminary evidence of their potential clinical and research value as diagnostic biomarkers. Further studies are needed to clarify the clinical significance of the observed relationships, particularly since the CFS condition has not been clearly associated with biomarkers, unlike many other pathophysiological disease states.
Download Provisional PDF Here
Article Type: Research Article
Citation: Kubow S, Sorenson M, Alkazemi D, Roberts JL II, Adamski KN, et al. (2015) Novel Associations of F2-Isoprostanes, F3-Isoprostanes and Isofurans in Older Adults with Chronic Fatigue Syndrome: An Exploratory Study. Clin Res Open Access 1(1): doi: http://dx.doi.org/10.16966/2469-6714.103
Copyright: © 2015 Kubow S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access