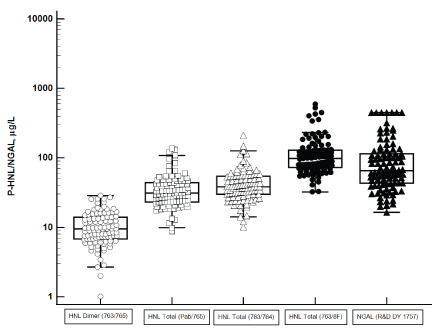
Figure 1: The comparison of HNL/NGAL assays. Four HNL assays with different antibody configurations and one commercial NGAL assay are shown. Plasma samples were obtained from patients at admission to the ICU (Day 1).

Per Venge1,2* Shengyuan Xu1,2 Christer Peterson2 Ann-Katrin Eriksson2 Michael Hultström3,4 Anders Larsson1 Miklos Lipcsey4,5 Sara Bülow5 Robert Frithiof5
1Department of Medical Sciences, Clinical Chemistry, Uppsala University, Uppsala, Sweden*Corresponding author: Per Venge, Department of Medical Sciences, University of Uppsala, Uppsala, Sweden, Phone: +46705349646; E-mail: per.venge@medsci.uu.se
Background: A serious consequence of respiratory infections with SARS-CoV-2 is Acute Kidney Injury (AKI). The aim of this study was to evaluate some novel biomarkers measured in plasma for their associations with the outcome of this infection as to AKI and mortality.
Methods: Four different HNL (Human Neutrophil Lipocalin) assays were constructed using different antibody configurations, reflecting both neutrophil and epithelial cell activities. TK-1 (Thymidine kinase-1) was measured as a sign of cell destruction and proliferation. For comparison the plasma concentrations of NGAL (Neutrophil Gelatinase Associated Lipocalin), the cytokines IL-6, IL-8 and IFN-γ and of cystatin C, and procalcitonin were also measured. One hundred and three patients with severe respiratory failure admitted to the ICU were sampled for blood at admittance and at day three (n=67) after admittance.
Results: Sixty-five percent of the patients developed AKI. The performance of the HNL assay 763/8F was most closely associated to outcome as to AKI and mortality with Area Under the Receiver Operating Characteristics Curve (AUC) of 0.87 and a sensitivity of 80% and a specificity of 88% in COVID-19 patients with and without severe AKI (p<0.0001). TK-1 was significantly related to the development of AKI (p=0.01) and the three cytokines were related to mortality (p=0.004-p<0.001).
Conclusion: The HNL variant 763/8F and TK-1 are novel biomarkers that might become useful and novel tools in the management of COVID-19 patients, but likely also in other patients affected by AKI.
Serious consequences of respiratory infections with the SARSCoV-2 virus are Acute Kidney Injury (AKI) and ultimately death, as was frequently seen during the COVID-19 pandemic [1]. During the pandemic the Intensive Care Units (ICU) were overburdened by patients with this infection and respiratory failure. Numerous studies were carried out during the pandemic in the attempt to understand the pathophysiology of the organ injuries and also to identify biomarkers that might predict outcome as to the development of AKI [2-4]. Our own studies showed very clearly the involvement of neutrophils in the process [5-7]. Thus, the measurement of the activity of neutrophils or the concentrations in plasma of the neutrophil specificform i.e., the dimeric form, of Human Neutrophil Lipocalin (HNL) were clearly associated with the outcome of the patients with respect to the development of AKI and survival.
HNL, also called NGAL (Neutrophil Gelatinase Associated Lipocalin) or lipocalin 2 exists in several forms with different cellular origins [5,8-10]. The dimeric form (45 kD) seems to exclusively originate from neutrophils whereas other forms such as the monomeric HNL (22kD) in addition originates from epithelial cells [11]. It is also known that the different HNL forms may bind to and form complexes with many different known or unknown proteins (>90 kD) one of which is MMP9 (Matrix Metallo Proteinase 9 or Gelatinase) hence the name NGAL [12]. Thus, the presence in biological fluids of HNL may have several cellular origins and reflect many different processes. During the years we have developed polyclonal and monoclonal antibodies against the native variants of HNL and by this means created unique panels of antibodies with diverse epitope specificities. The combinations of these antibodies in our ELISAs (Enzyme-Linked Immunosorbent Assay) have provided us with unique tools for the investigation of the usefulness of these assays for the purpose of prediction, diagnosis and monitoring in human disease.
In this study we investigated the association of four different HNL assay variants to the outcome, as to AKI and survival, in a cohort of COVID-19 patients admitted to the ICU. For comparison we include in this report the results of the measurements of other potentially useful and interesting biomarkers such as some cytokines, NGAL, Procalcitonin and Thymidine Kinase 1 (TK1) [13].
Data found in this research are part of the PronMed study approved by the National Ethical Review Agency (Dnr 2017/043, with amendments 2020-01623, 2020-02719, 2020-05730, 2021-01469, and 2022-00526-01) and listed at ClinicalTrials.gov (NCT03720860). Informed consent was obtained from the patient or next of kin. The Declaration of Helsinki and its subsequent revisions were followed.
The study included 103 patients admitted to the ICU (Intensive Care Unit) of Uppsala University Hospital (Day 1). In 67 of the patients a second blood specimen (EDTA-plasma) was obtained at day 3. Data with regards to HNL from these patients have been presented previously and detailed information of the patient demographics is given in that publication [5].
Clinical data was collected from the electronic medical records. AKI severity was stagged according to Kidney Disease: Improving Global Outcome (KDIGO) creatinine criteria and renal replacement requirement solely [14].
Four different HNL assays were used and developed by Diagnostics Development, Uppsala, Sweden. Three HNL assays used plates coated with the HNL monoclonal antibody (mab) 763 and one with plates coated with the polyclonal antibody (pab) raised against dimeric HNL. The detecting antibodies were respectively mab 765, mab 764, mab 8F and mab 765. Normal concentrations of HNL were for mab763/ mab765:34µg/L (30-41 µg/L, IQ-range), for mab763/mab764: 34µg/L (30-41µg/L), for mab 763/8F: 48µg/L (39-55µg/L) and for pab/mab765: 36 µg/L (31-42 µg/L).
The measurement of NGAL was performed by the ELISA kitDY1757 purchased from R&D systems, Minneapolis, MN, USA.
The Bioplex assays were used for the measurement of IL-6, IL-8 and IFN-γ using a Luminex MagPix instrument (Bio-Rad Laboratories AB, Sundbyberg, Sweden).
Thymidine Kinase 1 was measured by the AroCell TK 210 ELISA kit generously provided by Arocell AB, Uppsala, Sweden.
All above biomarkers were measured according to the instructions of the manufacturer and with a CV% of duplicate samples of <10%. All clinical data were blinded to the analyzing personal.
Routine chemistry, including inflammatory markers such as C - reactive protein (CRP), Procalcitonin (PCT) and Cystatin C were performed in the hospital central laboratory
Non-parametric statistics was applied throughout unless otherwise indicated. For comparison between independent results MannWhitney U test was used and for the comparison of the results of multiple groups Kruskal-Wallis ANOVA was used. Correlations between biomarkers were calculated by Spearman rank correlations. The diagnostic performances of the assays were estimated by ROC analyses. The statistical programme Medcalc was used in all calculations: MedCalc® Statistical Software version 20.106 (MedCalc Software Ltd, Ostend, Belgium; https://www.medcalc.org; 2022).
The clinical characteristics of the 103 patients included in the investigation have been previously published [5]. Briefly, all patients were adult ICU patients, admitted to the ICU during the first wave of the pandemic 10 (SD 5) days after the initial symptoms of COVID-19. The average age was 60.5 years (SD 14.1) and 74% were male. The most common comorbidity was hypertension. Invasive ventilation was used for 60% of the patients and 30-day mortality was 24%. The diagnostic criteria for AKI using changes in plasma creatinine was reached in 63% (30% stage I, 17% stage II and 17% stage III). No patients were treated with corticosteroids for COVID-19.
The antibody configurations of HNL immunoassays vary, which give rise to substantial differences in the performances of the assays. Figure 1 shows the concentrations measured in plasma of HNL and NGAL. In the plasma assays of HNL the same calibrator i.e. native dimeric HNL, was used whereas the calibrator used in the R&D NGAL assay was the recombinant, monomeric protein. By the use of the monoclonal antibodies 763/765 the assay specifically measures the dimeric form of HNL and as can be seen in the figure the plasma concentrations of HNL using this assay were 5-10 times lower than any of the other formats, which identify several forms of HNL/NGAL. The highest concentrations were measured by the 763/8F configuration. In spite of differences in measured concentrations three of the HNL assays were highly and linearly correlated (Figure 2a) whereas the correlation to NGAL was much lower, which is also illustrated in the figure 2b. Also, the correlation between the three HNL assays measuring most forms of HNL in plasma and the dimer assay was substantially lower than between the three HNL assays measuring “total” concentrations of HNL. In the further evaluation of the performances of the HNL/ NGAL assays we will report the performances of the NGAL (R&D) and HNL (763/8F) assays.

Figure 1: The comparison of HNL/NGAL assays. Four HNL assays with different antibody configurations and one commercial NGAL assay are shown. Plasma samples were obtained from patients at admission to the ICU (Day 1).
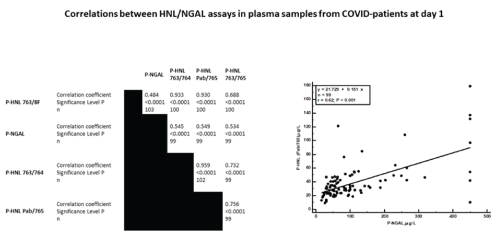
Figure 2: The correlations between HNL and NGAL assays. Three HNL assays with different antibody configurations and one commercial NGAL were compared. Plasma samples were obtained from patients at admission to the ICU (Day 1). Spearman rank correlation was used, and the correlation coefficients, p-values and numbers (n) are all given in the figure. The figure also includes a graph in which the comparison between plasma concentrations of NGAL are compared to plasma concentrations of HNL as measured with the configuration of pab/765. The statistics in the graph were calculated by linear regression analysis. The r-value and the equation of the regression analysis are given in the figure.
The clinical performances of the HNL/NGAL assays are illustrated in figure 3 in which HNL/NGAL plasma concentrations were measured in COVID patients on their admission samples to the ICU. The relation to different stages of AKI disease to plasma concentrations of HNL showed progressive increments of HNL by higher stages of AKI according to the KDIGO definition (p=0.00006, Kruskal-Wallis). This relation, however, was absent when plasma concentrations of NGAL were measured and marginally significant when measured by the HNL Dimer assay (p=0.02, Kruskal-Wallis) (not shown).
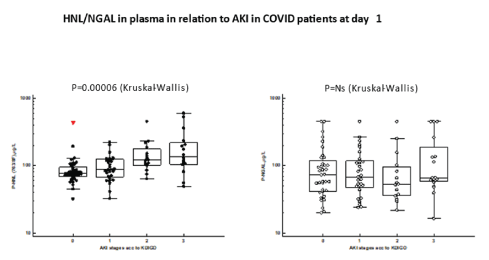
Figure 3: The plasma concentrations of HNL 763/8F and NGAL at admission day to ICU (Day 1) are shown in relation to AKI stages according the KDIGO classification. Differences between plasma concentrations were calculated by Kruskal-Wallis ANOVA and the statistics given in the figure.
In table 1, we show for comparison the differences in plasma concentrations the selected biomarkers in relation to severe AKI. HNL concentrations were strongly related to the development of severe AKI, but also some of the other selected biomarkers such as Cystatin C, IL-6, IFN-g and TK-1 were significantly higher in severe AKI. Cystatin C showed as expected a progressive increment in relation to increasing stages of AKI, whereas HNL Dimer, IL-6 and IFN-g only showed raised levels in plasma of patients with AKI stage 3 i.e. in severe AKI, but not in AKI stages 1-2. TK-1 showed progressive increments in relation to AKI as shown in figure 4 (r=0.36, p=0.004). The TK-1 concentrations in plasma samples from patients with AKI stage 0 were significantly higher than concentrations found in healthy non-covid subjects (<0.45µg/L), p<0.0001.
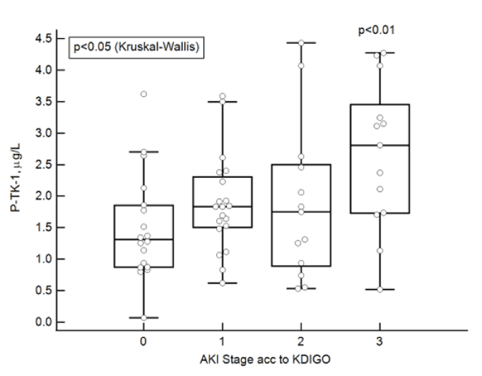
Figure 4: The plasma concentrations of TK-1 at admission in relation AKI stages according to the KDIGO classification. Differences were calculated by Kruskal-Wallis ANOVA and the statistics given in the figure. The comparison between concentrations in plasma between stages 0 and 3 are also given and calculated by Mann-Whitney U-test.
| AKI stage 3: Yes | AKI stage 3: No | ||||
| Variable | n | Median | n | Median | P |
| P-CRP, mg/L | 18 | 182 | 92 | 168 | Ns |
| P-Procalcitonin, µg/L | 16 | 0.99 | 87 | 0.41 | Ns |
| P-Cystatin C, mg/L | 13 | 1.83 | 67 | 1.08 | 0.004 |
| P-HNL Dimer, µg/L | 17 | 15.2 | 80 | 8.43 | 0.002 |
| P-HNL (763/8F), µg/L | 17 | 143.1 | 81 | 83.6 | 0.0008 |
| P-NGAL (R&D DY1757), µg/L | 16 | 65.5 | 81 | 61.6 | Ns |
| P-TK-1, µg/L | 15 | 2.80 | 50 | 1.67 | 0.02 |
| P-IL-6, ng/L | 18 | 59.9 | 87 | 25.1 | 0.02 |
| P-IL-8, ng/L | 18 | 16.5 | 89 | 14.7 | Ns |
| P-IFN-g, ng/L | 18 | 24.9 | 89 | 15.8 | 0.003 |
Table 1: Plasma concentrations at admission day in patients who developed AKI stage 3 or not. Mann-Whitney’s non-parametric test was applied for the comparison between groups.
The selected plasma biomarkers were further evaluated in relation to survival as shown in table 2. Plasma concentrations of HNL (763/8F) and of Dimeric HNL were both related to survival for 30 days as were the three cytokines, whereas no significant differences were seen for NGAL, TK-1, PCT, Cystatin C or CRP. Highly significant correlations were seen between IFN-g and the two HNL assays, but only in plasma of survivors (r=0.33-0.39, p=0.004-0.0007, Spearman Rank).
| Alive 30 days: No | Alive 30 days: Yes | ||||
| Variable | n | Median | n | Median | P |
| P-CRP, mg/L | 27 | 155 | 83 | 177 | Ns |
| P-Procalcitonin, µg/L | 24 | 0.61 | 79 | 0.44 | Ns |
| P-Cystatin C, mg/L | 19 | 1.43 | 61 | 1.05 | 0.0001 |
| P-HNL Dimer, µg/L | 24 | 13.6 | 73 | 8.68 | 0.009 |
| P-HNL (763/8F), µg/L | 24 | 126.9 | 74 | 81.7 | 0.0001 |
| P-NGAL (R&D DY1757), µg/L | 24 | 88.4 | 73 | 58.3 | Ns |
| P-TK-1, µg/L | 14 | 2.06 | 51 | 1.77 | Ns |
| P-IL-6, ng/L | 24 | 47.4 | 81 | 23.2 | 0.004 |
| P-IL-8, ng/L | 24 | 24.7 | 83 | 13.0 | 0.0002 |
| P-IFN-g, ng/L | 24 | 23.9 | 83 | 14.7 | 0.009 |
Table 2: Plasma concentrations at admission day in patients who survived 30 days at ICU or not. Mann-Whitney’s non-parametric test was applied for the comparison between groups.
The performances of the studied biomarkers were evaluated by ROC-analysis. Figure 5 show the ROC-analysis in relation to the development of severe AKI. For the prediction of severe AKI the AUC of the HNL assay (763/8f) was significantly raised and slightly higher than the AUC of the Dimeric HNL assay and clearly superior to the NGAL assay. However, also plasma concentrations of IL-6, IFN-g and TK-1 were significantly related to the development of severe AKI.
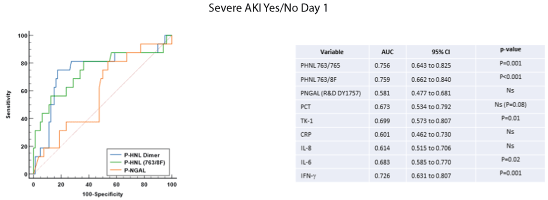
Figure 5: The receiver operating characteristics (ROC) curves, areas under the curve (AUC) are shown for the two HNL assays, HNL Dimer (763/765) and HNL 763/8F at admission day to the ICU (Day 1) and NGAL in relation severe AKI (AKI stage 3), yes or no. For comparison the AUCs of several other biomarkers are shown for comparison.
As predictors of survival for 30 days the HNL 763/8f assay showed the highest performance together with the Dimeric HNL and the three cytokines IL-6, IL-8 and IFN-g (Figure 6), whereas non-significant performances were seen for NGAL, TK-1, PCT and CRP.
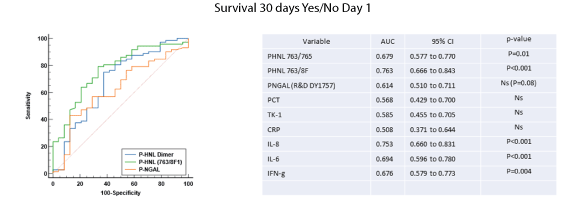
Figure 6: The receiver operating characteristics curves, areas under the curve (AUC) are shown for the two HNL assays, HNL Dimer (763/765) and HNL 763/8F at admission day to the ICU (Day 1) and NGAL in relation to survival 30 days, yes or no. For comparison the AUCs of several other biomarkers are shown for comparison.
The performances of HNL/NGAL-assays were further evaluated at day three after admission and shown in figures 7a and 7b. The relationship to severe AKI and survival for 30 days increased significantly for the HNL/NGAL assays except for the HNL Dimer assay when measured as AUC of the ROC-curves. The performance of the NGAL assay also increased at day three but was significantly inferior to the HNL 763/8F assay in relation to severe AKI as shown in the figure 7 (p=0.03). In relation to survival 30 days no significant differences were seen between the HNL/NGAL assays.
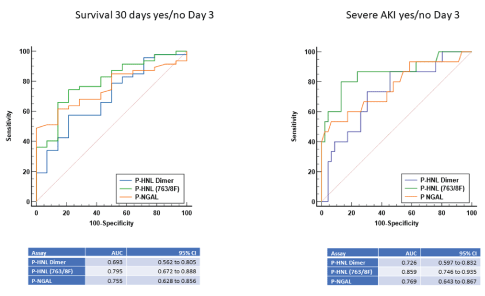
Figure 7: The comparison of AUCs by ROC analysis of the associations to survival 30 days or severe AKI of plasma concentrations of the two HNL assays, HNL Dimer and HNL 763/8F and NGAL. The samples were obtained day 3 after the admission to ICU.
The relation of HNL/NGAL assays at day three to stages of AKI according to KDIGO are shown in figure 8 and show a closer relationship of HNL (763/8F) to KDIGO (p<0.0001) than plasma concentrations measured by the NGAL assay (p=0.0008).
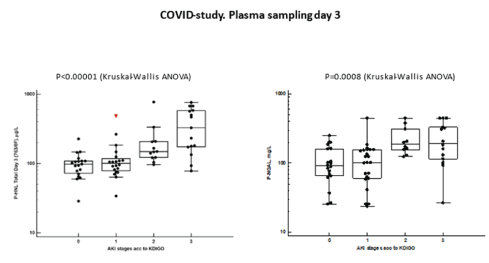
Figure 8: The plasma concentrations of HNL 763/8F and NGAL at day 3 after admission to ICU are shown in relation to AKI stages according the KDIGO classification. Differences between plasma concentrations were calculated by Kruskal-Wallis ANOVA and the statistics given in the figure.
The main message of this report is the demonstration of the important differences in performances of the various HNL/NGAL assays related to their antibody configurations. This was related to both differences in measured plasma concentrations of HNL/ NGAL, but more importantly also to their clinical performances. The differences between the concentrations measured by the Dimeric HNL assay and the other HNL assays are well explained by the fact that this assay only detects dimeric HNL in plasma, which is 10-20% of the total concentration of circulating HNL. The fact that the HNL assay 763/8F measured 2-4 fold higher concentrations than the pab/765 and mab763/764 assays was unexpected and is likely explained by the fact that additional epitopes are detected by the antibody combination of mab763/8F. This quality of the 763/8F assay also translated into slightly better clinical performance as judged by the relation to stages and severity of AKI (data not shown). However, all HNL assays, but the dimeric HNL assay, performed significantly better than the NGAL assay with closer associations to outcomes such as to severe AKI and mortality which probably emphasizes the importance of detecting the main HNL/NGAL molecules in plasma related to the development of AKI. These results are in accordance with a previous report where urinary NGAL did not robustly predict KDIGO stage in critically ill COVID-19 patients [15]. It was also suggested from our findings that the major quality of the HNL 763/8F assay may be the unique reflection of the processes involved in the development of AKI. In this respect the sensitivity and specificity of HNL 763/8F in the detection and diagnosis of AKI at day 3 after admission were80 and 88 %, respectively, as compared to NGAL with 53% sensitivity and 90% specificity, respectively. It should also be pointed out that the raised concentrations of HNL 763/8F most likely are consequences of processes in the epithelial cells and not a consequence of the virus infection per se, since other studies showed normal plasma concentrations of HNL in other serious virus infections[16-19]. Also, HNL concentrations in our patients not having AKI were similar to those of healthy non-infected subjects in spite of those patients being admitted to the ICU because of COVID-19 with serious respiratory symptoms. The lung and intestinal epithelial cells are rich sources of HNL (The Human Protein Atlas) and a contribution to the raised plasma concentrations from these cells cannot be excluded. Possibly epithelial injury is a common phenomenon affecting all these organs in parallel. Others have argued that raised lipocalin2/NGAL/HNL concentrations in plasma originate mainly from neutrophils, which we question based on our findings in this report [20], but nevertheless find the raised concentrations closely related to the outcome of the critically ill COVID-19 patients.
As shown before, the plasma concentrations of cytokines were associated to both AKI and survival [5]. Overall, though, the strongest association seemed to be to mortality, which is in line with previous findings and the notion of a cytokine storm [21,22]. However, the finding of the association of Thymidine Kinase 1 to stages of AKI but not survival is novel. Previous studies showed that plasma concentrations of TK-1 are elevated in many viral infections and the finding of raised concentrations in SARS-CoV-2 infections in this report may be in line with this notion [17]. TK-1 is usually used as a marker of tumour growth and the question arises whether the progressive increments related to AKI stages reflect tissue injury or expansion [23-25]. In a logistic regression analysis with the inclusion of all biomarkers described in this report, TK-1 together with HNL 763/8F were the only two independent biomarkers of severe AKI (data not shown). Even if the number of observations is relatively low it further emphasizes the potential of TK-1 as an interesting biomarker in COVID-19.
The limitations of this study are the relatively small number of patients who were developing AKI and died from there infection. The results presented in this study should therefore be confirmed in larger studies on patients who develop AKI due to COVID-19 or any other cause.
We conclude from this study that plasma concentrations of HNL/ NGAL are closely related to AKI and mortality in COVID-19 patients, but that differences in antibody configurations of the assays show differences in their clinical performances with the HNL 763/8F assay as the superior assay. Most likely the concentrations of HNL as measured by this assay in plasma of COVID-19 patients uniquely reflect epithelial cell injury such as seen in AKI. This assay may prove useful as a novel tool in the management of such patients, but also in other patients affected by AKI. Future studies will also show whether the HNL 763/8F assay is suitable for the detection of Chronic Kidney Injury (CKI). Understanding of the actual molecular variants of HNL that are detected by this assay should be an important issue for future studies. However, studies on larger cohorts of patients with AKI as the consequence of COVID-19 or other causes should be performed and confirm our findings in this report before the HNL 763/8F assay can be recommended for clinical use.
We thank the study nurses Joanna Wessbergh and Elin Söderman for their expertise in compiling the study. We are also indebted to the biobank research assistants Erik Danielsson, Labolina Spång, Amanda Svensson and Philip Karlsson for organizing the sample analysis and the Uppsala Intensive Care COVID-19 research group: Tomas Luther, Anders Larsson, Katja Hanslin, Anna Gradin, Sarah Galien, Sten Rubertsson and Jacob Rosén for patient inclusion.
The Swedish Research Council (R.F.: 2014-02569, 2014-07606), SciLifeLab/Knut and Alice Wallenberg national COVID-19 research program (M.H.: KAW 2020.0182, KAW 2020.0241), the Swedish Heart-Lung Foundation (M.H.: 20210089, 20190639, 20190637) and the Swedish Kidney Foundation (R.F.: F2020-0054). Funding bodies had no role in the design of the study, data collection, interpretation, or in the writing of the manuscript.
Per Venge planned and wrote the report, Shengyuan Xu, Christer Peterson, Ann-Katrin Eriksson were instrumental in the development of the HNL assays and supervised the laboratory work, Anders Larsson was responsible for the NGAL assay, Michael Hultström, Miklos Lipcsey, Sara Bülow and Robert Frithi of were responsible for patient recruitment and clinical characterization of the patients. All co-authors read and approved the report.
Download Provisional PDF Here
Article Type: RESEARCH ARTICLE
Citation: Venge P, Xu S, Peterson C, Eriksson AK, Hultström M, et al. (2022) Plasma Biomarkers Associated to Outcome in Patients with SarsCov-2 Infection. J Clin Lab Med 7(1): dx.doi.org/10.16966/2572-9578.141
Copyright: © 2022 Venge P, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access