
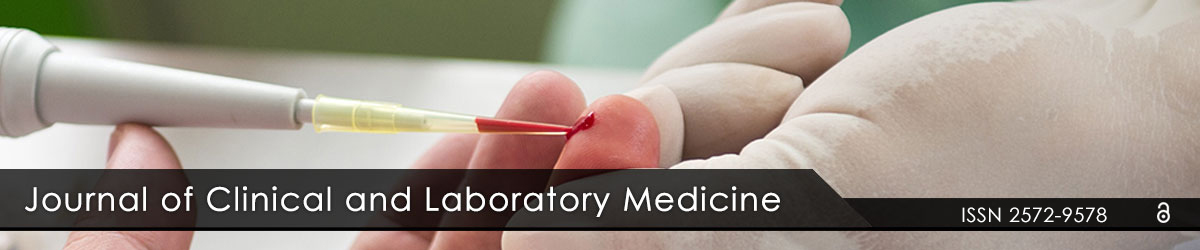
Figure 1: The erythroid precursors show dysplastic morphology (white arrow), with nuclear and cytoplasmic vacuolization (black arrow) and nuclear to cytoplasmic asynchrony (red arrow). Leishman × 40X.

Kafil Akhtar1* Sadaf Haiyat2 Asim I Khan2 Binjul Juneja3 Taiba Khan4 Arif SH1
1Professor, Department of Pathology, Jawaharlal Nehru Medical College, Aligarh Muslim University, Uttar Pradesh, India*Corresponding author: Kafil Akhtar, Professor, Department of Pathology, Jawaharlal Nehru Medical College, Aligarh Muslim University, Uttar Pradesh, India, E-mail: drkafilakhtar@gmail.com
AML with myelodysplasia related changes is an uncommon type of acute myeloid leukemia. It comprises less than 5% of acute leukemias. According to the recent World Health Organization (WHO-2016) classification, AML cases with ≥ 50% or more erythroid cells and ≥ 20% total myeloblasts should be diagnosed as AML with myelodysplasia-related changes. Morphologic cellular features help to establish the diagnosis. We present a rare case of AML with myelodysplasia related changes in a 35-year-old female who presented with low-grade fever, mild epistaxis, and shortness of breath and diffuse sternal tenderness. The peripheral smear showed features of pancytopenia with 6% blasts along with evidence of hemolysis. Bone marrow examination revealed erythroid hyperplasia with 65% erythroblasts and 24% myeloblasts. Flow cytometry was used for the confirmation of the diagnosis. The patient was administered chemotherapy with Azacitidine 75 mg/m2/day × 7 days in IV infusion along with 2 units of red cell concentrate prophylactically to prevent anemia. Molecular studies are needed to understand better the pathogenesis of AML with myelodysplasiarelated changes and to develop newer diagnostic and prognostic markers.
AML with myelodysplasia related changes; Erythroblasts; Bone marrow examination; Flow cytometry
AML with myelodysplasia related changes is a rare form of the disease, characterized by uncontrolled proliferation of erythroid and myeloid precursors. It accounts for 3-5% of all de novo Acute Myeloid Leukemia (AML) cases world-wide [1-3]. With myelodysplasia related changes is more common in the old age but can occur at any age, including childhood [2,4].
Coppelli, in 1912, described features of anemia and splenomegaly in a patient with foci of erythroblast in the liver, spleen, lymph nodes and bone marrow, but without circulating blasts and named it Erithromatosis [4]. Giovanni Di Guglielmo, reported a case of acute erythremic myelosis in 1917, with immature erythroid and myeloid elements [5]. Moeschlin, et al. described the term acute erythroid leukemia in 1940 [6]. In 1969 Dameshek W, et al. [7] described three phases of the disease in the bone marrow: an erythemic phase, an erythromyeloblastic phase and myeloblastic phase [4,7].
The recent World Health Organization (WHO-2016) classification was formulated by the Clinical Advisory Committee which agreed to keep the AML, NOS subcategories with only a single change [8,9]. The subcategory of acute erythroid leukemia, erythroid/myeloid type (previously defined as a case with ≥ 50% BM erythroid precursors and ≥ 20% myeloblasts among non-erythroid cells) has been removed from the AML category. In the new classification, myeloblasts are always counted as a percentage of total marrow cells and the majorities of such cases have <20% total blast cells and are now classified as MDS (usually MDS with excess blasts). This change was based on the close biologic relationship of erythroid/myeloid type acute erythroid leukemia to MDS in terms of its clinical presentation, morphologic features, and genetic abnormalities, as well as the low reproducibility of non-erythroid blast counts and an attempt to achieve uniformity in expressing blast percentages across all myeloid neoplasms [10-14].
Cases with ≥ 50% or more erythroid cells and ≥ 20% total myeloblasts usually meet criteria for AML with myelodysplasiarelated changes and should be diagnosed as such; cases with ≥ 20% total myeloblasts not meeting criteria for AML with myelodysplasiarelated changes or AML with recurrent genetic abnormalities should be categorized as one of the other subtypes of AML, NOS. Pure erythroid leukemia remains as an AML, NOS subtype and is now the only type of acute erythroid leukemia [8,9].
The diagnosis of AML with myelodysplasia related changes is based on the morphologic examination of the peripheral blood smear and bone marrow and flow cytometry for confirmation. Treatment modality in the form of chemotherapy and bone marrow transplantation leads to long term survival of the patient. But this disease is characterized by poor remission rate and short survival [7].
A 35-year-old woman presented to the medical clinic with fatigue, generalized weakness, abdominal discomfort and loss of appetite for 6 weeks. For the last 4 days, she had also complaints of low grade, continuous fever without chills and rigor, mild epistaxis, shortness of breath and mild sternal tenderness. There was no history of weight loss, smoking, alcohol intake and toxin exposure or drug abuse. Past history, menstrual history and family history were non-contributory.
On examination, she had moderate pallor and mild dyspnea with a regular respiratory rate of 20 per minute. The oral temperature was raised to 101°F. She had no lymphadenopathy, petechiae, gum bleeding or bony tenderness. On abdominal examination, there was mild hepatosplenomegaly, with no clinical abnormality in other systems. The patient was admitted for further workup.
Routine haematological investigations revealed pancytopenia with a total leucocyte count of 2.8 × 109/L, red cell count of 2.9 million mcL, platelet count of 350 × 109/L, haemoglobin 5.5 gm/ dl and hematocrit 22.5 percent. The peripheral smear examination showed severely hypochromic red blood cells with mild to moderate anisopoikilocytosis with few target cells, spherocytes, schistocytes and several polychromatic cells. There were 6 nucleated red blood cells per hundred white blood cells. The differential leucocyte count revealed 38% neutrophils, 49% lymphocytes, 3% eosinophils, 4% promyelocytes and 6% blasts. The reticulocyte count was 5.5 percent. The erythrocyte sedimentation rate was 18 mm in the first hour. Biochemical investigations showed normal renal and liver function tests, with elevated serum lactate dehydrogenase (340 U/L) and uric acid levels (12 mg/dL).
Bone marrow aspirate smear was hypercellular with erythroid hyperplasia with marked megaloblastic features. Erythroblasts comprised 65% of all the nucleated cells of the marrow. The myeloid precursors in the form of myeloblasts were large cells with increased nucleo-cytoplasmic ratio, coarse chromatin and basophilic cytoplasm and erythroid precursors showed dysplastic morphology with nuclear and cytoplasmic vacuolization and nuclear to cytoplasmic asynchrony (Figure 1). Myeloblasts comprised 24% of non erythroid cells and were strongly myeloperoxidase positive (Figure 2). Periodic-Acid Schiff stain (PAS) showed diffuse positivity in erythroblasts (Figure 3). Megakaryocytes, lymphocytes and plasma cells were normal both in number as well as morphology. Few ringed sideroblasts were seen on Perls’ staining. Both erythroblasts and myeloblasts showed mitotic activity. Flow cytometry was performed on the bone marrow sample to confirm the diagnosis. CD33 depicted dim positivity with heterogenous positivity for CD117 led to confirm myeloid nature of the blast cell population. CD45 dim to negative and CD235a (Glycophorin A) positive expression were used to confirm erythroid nature of the blast cells (Figures 4 and 5). B and T cell markers were negative. Karyotyping revealed monosomy 5 or del (5q) (Figure 6). A final diagnosis of AML with myelodysplasia related changes was given. The patient was administered chemotherapy with Azacitidine 75 mg/m2/day × 7 days in IV infusion along with 2 units of red cell concentrate prophylactically to prevent anemia. Treatment was well tolerated by our patient except for mild neutropenia, and she is doing well after 6 months of follow up period.

Figure 1: The erythroid precursors show dysplastic morphology (white arrow), with nuclear and cytoplasmic vacuolization (black arrow) and nuclear to cytoplasmic asynchrony (red arrow). Leishman × 40X.
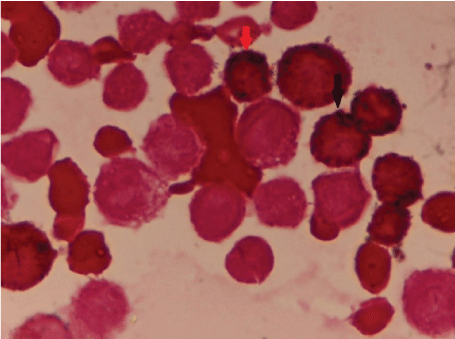
Figure 2: Myeloblasts comprised of 24% of non erythroid cells and showed strong cytoplasmic myeloperoxidase positivity (red and black arrow). Myeloperoxidase × 40X.
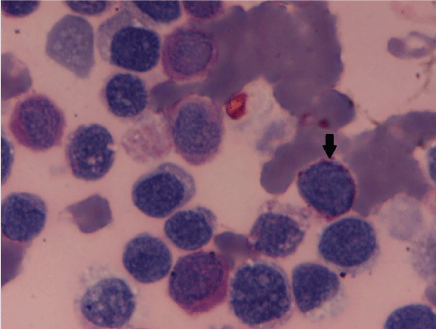
Figure 3: Periodic-acid schiff stain showed diffuse cytoplasmic block positivity in erythroblasts (black arrow). PAS × 40X.
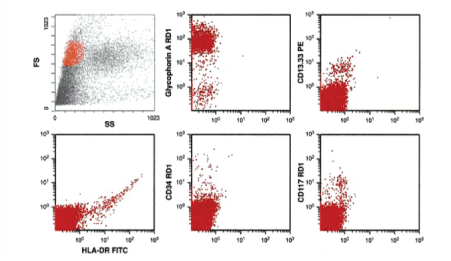
Figure 4: Flow cytometry analysis of the bone marrow revealed CD117 and CD33 positivity to confirm myeloid nature of the blast cell population and CD45 dim to negative and Glycophorin A(CD235a) positivity to confirm erythroid nature of the blast cells.
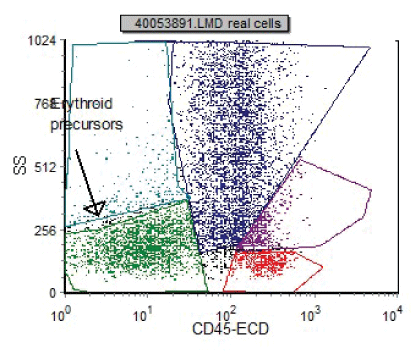
Figure 5: Flow image shows CD45 dim to negative staining to confirm the erythroid nature of the blast cells.
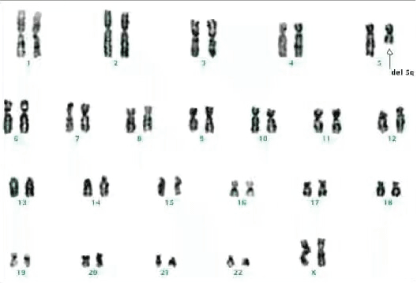
Figure 6: Karyotyping studies revealed monosomy 5 or del (5q).
AML with myelodysplasia related changes is a heterogeneous disease, which may arise de novo or secondary to cytotoxic chemotherapy. It is usually seen in the elderly beyond the fifth decade of life. The disease has a bimodal distribution: below 20 years of age and in the seventh decade [2]. Few cases have also been reported in childhood [2,4]. AML with myelodysplasia related changes has a male preponderance [3]. The present case is a rare presentation in a 35-yearold female.
The clinical features of AML with myelodysplasia related changes are very non-specific. Common signs and symptoms include pallor, fever, and hepatosplenomegaly. Nearly one-third of the patients present with hemorrhage and 20-40% show hepatosplenomegaly [15]. Patients become symptomatic early within one to two months after the onset of symptoms [15].
The diagnosis of AML with myelodysplasia related changes requires morphologic examination of the peripheral blood smear and bone marrow followed by flow cytometry for confirmation. The peripheral blood smear examination can sometimes be misleading because they show pancytopenia but are often devoid of blasts in up to fifty percent of the patients. In the present case, only 6% of blasts were seen on peripheral smear. Nonspecific red blood cell abnormalities like anisocytosis, poikilocytosis, anisochromia, polychromasia and basophilic stippling may be seen [8].
The bone marrow shows hypercellularity with dysplastic haematopoietic precursor cells. Erythroid hyperplasia is seen with medium to large erythroblasts having basophilic cytoplasm and megaloblastic nucleus i.e., asynchronous nucleocytoplasmic maturation. The cytoplasm of the immature cells frequently shows vacuolations. Features of dyserythropoesis like multinucleation, budding and bizarre nuclear forms are often present. Megakaryocytes are often dysplastic, with abnormalities of nucleus segmentation or size [16,17]. In our case, bone marrow aspirate smear was hypercellular with erythroid hyperplasia with marked megaloblastic features, erythroblasts comprised of 65% of all the nucleated cells of the marrow and myeloblasts comprised of 24% of non erythroid cells and were strongly myeloperoxidase positive. Ring sideroblasts are seen in few cases on Perl’s stain. The atypical erythroblasts are negative for Myeloperoxidase and Sudan Black B stains but show coarse granular positivity with PAS staining. Immunophenotyping by flow-cytometry is superior to immunohistochemistry in AML with myelodysplasia related changes because a wider array of antibodies is available inflow for a conclusive diagnosis [3].
The frequent cytogenetic abnormalities seen in AML with myelodysplasia related changes are monosomy 5 or del (5q), monosomy 7 or del (7q) and trisomy 8. This disease is characterized by poor remission rate and poor prognosis with short survival [18,19]. Immunophenotyping is required for assessing the prognosis.
AML with myelodysplasia related changes is a rare type of acute leukemia with a poor prognosis and short survival. A thorough clinical history and haematological investigations with additional cytogenetic and molecular studies are required to reach a conclusive diagnosis.
Download Provisional PDF Here
Article Type: CASE REPORT
Citation: Akhtar K, Haiyat S, Khan AI, Juneja B, Khan T, et al. (2020) Acute Erythroid Leukemia: A Rare Case Report. J Clin Lab Med 5(1): dx.doi. org/10.16966/2572-9578.133
Copyright: © 2020 Akhtar K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access