Abstract
Background: Glycosylated Hemoglobin (HbA1c) is a marker for diabetes used to diagnose, monitor treatment compliance and overall management of a patient’s diabetes. Measured routinely in the clinical laboratory, HbA1c reflects the preceding three to four months average diabetic status of a patient. Because clinical laboratory protocol calls for the rejection of spun specimen from HbA1c level, this study investigates the effect of pre- and post-centrifugation on measured HbA1c values of whole blood specimens.
Methods: Whole blood specimen from normal, pre-diabetic and diabetic patients were drawn, processed and analyzed per laboratory standard operating procedure for HbA1c levels on three different laboratory instruments. The specimens were subsequently centrifuged, then re-suspended by rocking gently back and forth and reanalyzed on the same instruments. The pre and post centrifugation HbA1c values were compared and evaluated for differences using statistical analysis.
Results: The pre- and post centrifugation HbA1c values of whole blood were found to be statistically similar within each group (normal, pre-diabetic or diabetic). However, significant variation (p-values <0.05) of measured HbA1c values were observed between instrument analyzers.
Conclusions: Contrary to current laboratory protocol, specimen centrifugation does not affect HbA1c levels. The binding of glucose to hemoglobin is strong, stable and irreversible. Laboratory protocols that call for the rejection of spun whole blood specimen and subsequent redraw request of fresh specimen for HbA1c levels needs to be revised. Re-suspension of centrifuged whole blood samples can be processed for HbA1c levels without compromising patient result.
Abbreviations
HbA1c - hemoglobin A1c (glycosylated hemoglobin); ADA - American Diabetic Association; OGTT - oral glucose tolerance test; BNP - beta natriuretic peptide
Keywords
Whole blood; Diabetes; HbA1c; Centrifugation; Analyzers
Introduction
Diagnosis of diabetes is commonly done by measuring a patient’s blood glucose. While three of the four criteria directly measure the patient’s blood glucose, HbA1c measurement is indirect. It is the quantitative in-vitro measurement of the fraction of hemoglobin A that is bound to glucose or glycated to one or both N-terminal valines of the β-chain. Per the American Diabetes Association (ADA) [1], diabetes is diagnosed when (i) a patient’s fasting blood glucose levels is or exceeds 126 mg/dl, (ii) symptoms exist in a patient with blood glucose greater than 200 mg/dl, (iii) the 2-h post oral glucose tolerance test (OGTT) is or exceeds 200 mg/dl and/or (iv) HbA1c is greater than 6.4%. High blood glucose reflects an absolute or relative absence of insulin secretion or poor metabolic response to insulin action. HbA1c levels reflect a person’s diabetic status over the preceding three to four months. It is higher the longer red blood cells are in circulation exposed to higher glucose levels. Unlike regular and repeat daily monitoring of glucose levels, measurement of HbA1c is convenience for the patient and is relatively unaffected by acute (e.g., feeding, fasting, stress or illness related) perturbations in glucose levels [2].
Because a strong link exists between glucose levels and HbA1c, measured HbA1clevels on human whole blood can also indirectly be used to diagnose diabetes3. HbA1c level greater than 6.4% is diagnostic of diabetes per ADA criteria. In general, OGTT is more sensitive than HbA1c in the diagnosis of diabetes. Glycated hemoglobin is more specific in identify individuals at risk for developing diabetes as well as aid in the long term monitoring and management of blood glucose levels in diabetics [1-3].
Diabetes is the leading cause of blindness in working age Americans [4], the most common cause of end-stage renal disease in the United States as well as peripheral neuropathy in the Western world [4,5]. Higher HbA1c reflects poor control of blood glucose levels and increases the patient’s risk of developing microvascular complications [5]. A strong relationship has been documented between higher HbA1c levels and complications of diabetes such as retinopathy, nephropathy, neuropathy and microabuminuria [6-10]. The costs associated with diabetic complications can be significantly reduced with better control and management of a patient’s blood glucose levels [6, 9]. To do so, healthcare professionals frequently use HbA1c values to guide therapeutic decisions, assess quality of care, and predict risk for the development and progression of microvascular complications [5,10].
In determining HbA1c levels, current clinical laboratory protocol stipulates that whole blood specimens should not be centrifuged prior to measurements. That is specimens are routinely rejected and recollection requested if accidentally spun prior to re-suspension for HbA1c measurement. The whole blood specimen collected by venipuncture in a lavender top tube for HbA1c quantification can also be used for other tests; such as complete blood count (CBC), erythrocyte sedimentation rate (ESR) and/or beta natriuretic peptide (BNP) measurements. Generally, it is recommended that the CBC, ESR and/orHbA1c analysis be performed prior to BNP measurement.
BNP is measured from plasma; therefore the protocol for BNP measurement calls for the specimen to be centrifuged prior to testing. Once centrifuged for BNP analysis, the specimen is discarded and cannot be reused for HbA1c measurement. However, the rational for specimen rejection is tenuous and not based on scientific knowledge. Why centrifuged packed cells post BNP measurement cannot be re-suspended and mixed for HbA1c levels needed investigation. Therefore, the purpose of this study is to determine the effect of whole blood specimen centrifugation on HbA1c measurements. We hypothesized that centrifugation and resuspension of whole blood samples should not alter measured HbA1c levels because, as previously reported glycosylation of the N-terminal valines on the beta chains of hemoglobin are stable and irreversible [3].
Materials and Methods
Whole Blood specimen were collected by venous puncture into anticoagulant lavender top tubes and processed within 8 hours at room temperature or stored at 2-8°C for up to 7 days. Institutional Review Board (IRB) approval is filed with Winston-Salem State University IRB Compliance Officer in the Office of Sponsored Programs. All specimen for the study were left over clinical laboratory specimen destined for destruction or obtained from students’ volunteers practicing their phlebotomy skills in our clinical laboratory science program. The specimens were inspected to remove any visible fibrin clots and particulate matter using a clean applicator stick. All specimens were thoroughly mixed by rocking back and forth at low speed or gently inverted several times for homogeneity prior to analyzing on three different instrument analyzers (Architect 8100, Siemen DCA and Bayer A1C Now+) currently use in clinical laboratories or point-ofcare settings per the respective manufacturers’ package insert. Each instrument analysis was preceded with appropriate calibration and/or quality controls. After analysis, the specimen were centrifuged for 5 min at 6000 rpm, re-suspended by mixing as described prior to repeat measurement ofHbA1clevel on the analyzers.
Statistical analysis
The measured pre and post centrifugation HbA1c values were grouped into three pools of non-diabetic, pre-diabetic and diabetic per American Diabetes Association (ADA) classification [1]. Group 1: (HbA1c<5.7%- Non-diabetic), Group 2 (5.7-6.4, Impaired state or prediabetic) and Group 3 (>6.4 - Diabetic). All quantitative pre and post HbA1c data from each group were analyzed using IBM’s Statistical Package for the Social Sciences (SPSS) software to determine the mean and standard error. Independent T-test was used to test for differences between the measured pre and post centrifugation HbA1c values from each group and between instrument analyzers. P value <0.05 was considered to be statistically significant.
Results
The result show that glycosylated haemoglobin values from pre and post specimen centrifugation were similar irrespective of the analyser used in the measurement.HbA1c is a critical and routine test for the diagnosis and monitoring of treatment for diabetics. In an effort to understand the impact of specimen centrifugation on measured HbA1c levels, whole blood was collected and processed as described under methods. Measured HbA1c values from four initial samples were found to be similar before and after centrifugation. This observation was the first indication that glucose remained permanently bound to the N-terminal valine of the beta chain of haemoglobin [3] as previously reported and unaffected by specimen centrifugation.
To further demonstrate the validity of this finding, we analysed more specimens from each of the three ADA groups [nondiabetic (20 samples), pre-diabetic (16 samples) and diabetic (11 samples)] on the Architect 8100 system for mean and standard error. As seen in Figure 1, the pre and post specimen centrifugationHbA1c results for each group are statistically the same. To corroborate the Architect 8100 results, we repeated the analysis of more samples [nondiabetic (12 specimen), pre-diabetic (6 specimen) and diabetic (5 specimen)] on two other clinical laboratory analysers (Siemen DCA and Bayer A1C Now+) used for measuring HbA1c. The results in Figure 2, again show no statistical difference in pre and post centrifugation HbA1cvalues. We conclude, therefore that glycosylation of hemoglobin is stable and unaffected by centrifugation. These findings were equally true at much higher centrifugation speed. The observed variations in measured HbA1c values between instruments were expected because as previously reported, hemoglobin variants interfere with instrument sensitivity [3,11].
Because this investigation originated from the rejection of shared (HbA1c and BNP) specimen when accidentally spun for BNP levels prior to HbA1c measurement, we decided to test the impact of plasma volume on measured HbA1c levels. The whole blood specimen (two) were first processed and analysed on the architect for HbA1c value, spun and 500 µl of plasma removed (this amount was assumed to be the volume used for BNP measurement). The packed red cells were re-suspended by mixing as described and analysed again on the instrument. The reduction in plasma volume did not affect the whole blood pre and post centrifugation HbA1c measurements (data not shown). This observation indicates that the instrument analysers only measure the fraction of glycosylated red blood cells. Hence, HbA1c level is independent of plasma volume to which the red cells are suspended.
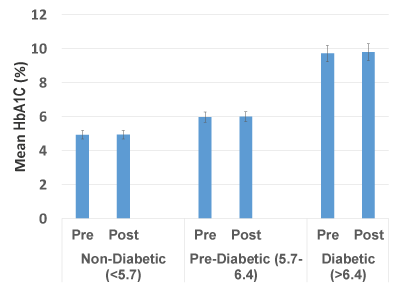
Figure 1: Comparison of the pre and post centrifugation of HbA1c value (mean ± SEM) calculated from specimens from each grouping analysed on Architect 8100 instrument show no statistical differences between pre and post centrifugation HbA1c measurements within groups. Nondiabetic (4.93 ± 0.011), Prediabetic (5.97 ± 0.023) and Diabetic (9.80 ± 0.063)
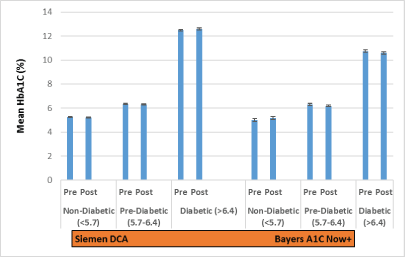
Figure 2: Average HbA1c measurements from two different instruments (Siemen DCA & Bayer’s A1C Now+) show no statistical difference (p-value less than 0.05) in group pre/post centrifugation HbA1c values comparison. The mean and standard error on Siemen DCA are: Nondiabetic (5.25 ± 0.029), Prediabetic (6.36 ± 0.042) and Diabetic (12.58 ± 0.070); and Bayer A1CNow + include: Nondiabetic (5.10 ± 0.122), Prediabetic (6.21 ± 0.071) and Diabetic (10.60 ± 0.106).
However, significant variation between instrument measurements were observed with p-values all less than 0.05 [Nondiabetic pre/post of 0.003 / 0.013, pre-diabetic pre/post of 0.038 / 0.004, and diabetic pre/post of 0.004 / 0.019].
Discussion
We have provided evidence that measured HbA1c values are not statistically affected by centrifugation of the whole blood specimens. The measured values were stable before and after centrifugation. Our finding agrees with previous study that glycosylation of the N-terminal valines of hemoglobin is stable and irreversible [2]. Once attached, the glucose remains bound to the hemoglobin throughout the lifespan (~4 months) of the red cell. Specimen rejection following centrifugation is premature and can be costly. Therefore, clinical laboratory procedures that call for rejection and redraw of spun specimen should be revised. Such revision would not compromise patient results and the quality of care. In fact, a revision should improve turn-around-time, patient care and reduce overall healthcare cost. Compared to those without diabetes, an individual diagnosed with diabetes at forty years old spends about $124,000 in medical expenditures on diabetes over their remaining lifetime [12]. Eliminating unnecessary specimen rejection should reduce costs especially for individuals and minority communities that are disproportionately affected by diabetes.
The benefits of HbA1c to patient care have been well documented. HbA1c (i) aids in the diagnosis of diabetes mellitus, (ii) helps identify patients who may be at risk for developing diabetes mellitus, and (iii) is use for the monitoring of long-term blood glucose control in individuals with diabetes mellitus. Furthermore, an accurate measurement allows the physician to help patients effectively manage long term microvascular complications with diabetes such as blindness, end-stage renal disease and nerve damage [7-10]. Such efforts should significantly reduce long term cost associated with the management of these chronic conditions.
Variations in HbA1c levels have been linked to several factors that affect red cell turnover rate such as patient age, race, ethnicity, hemoglobinopathies and coexisting medical conditions [1,11,13]. As demonstrated in our study, centrifugation does not affect the level of HbA1c glycosylation. Hemoglobinopathies and various hemoglobin variants cause major interference in analyzers and thus impacts HbA1c measurements [3]. In areas such as Africa and Southeast Asia, where there is a predominance of hemoglobin variants, fructosamine is the recommended alternative marker for diabetes [11] instead of HbA1c because glycemic control is needed over a shorter period of time. Fructosamine reflects the binding of glucose to plasma protein, mainly albumin which has a half-life of about two to three weeks [11].
Finally, because current laboratory practices rejects spun specimen for HbA1c measurement, we have provided new evidence to the contrary. Without compromising patient care, our finding demonstrates that spun samples can be re-suspended, analyzed and the results reported. It is supported by previously published results that the glucose-hemoglobin interaction is stable and irreversible [2]. Therefore, laboratory protocols that calls for rejection/redraw of post-centrifugation specimen needs to be revised. A revision should improve turn-around-time for better patient care; reduce cost associated with redraws and overall healthcare expenditures if unnecessary rejections of specimens are eliminated. The extent of the decrease in total healthcare expenditure requires further studies. Besides cost, patient exposure to risks associated with multiple blood collection such as injection pain, infections, and possibly hematoma are eliminated.
Article Information
Article Type: Research Article
Citation: Peterson S, Smith H, Cochrane S, Reid Q, Kenbata D, et al. (2018) The effects ofWhole Blood centrifugation on Hemoglobin A1c measurement. J Clin Lab Med 3(1): dx.doi. org/10.16966/2572-9578.118
Copyright: © 2018 Peterson S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
Received date: 15 Mar, 2018
Accepted date: 30 Mar, 2018
Published date: 05 Apr, 2018