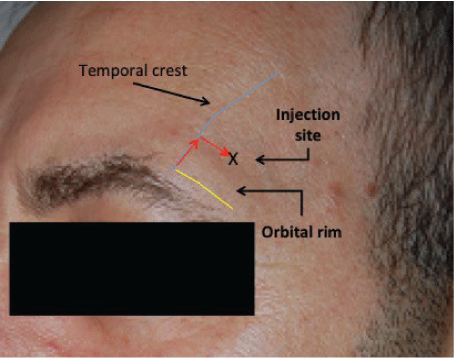
Figure 1: Site of HA injection over the temporal fossa.


Patrick Micheels1* Stéphanie Besse2 Badwi Elias3 Joan Vandeputte4
1Private office, Chemin de la Fontaine, Chêne- Bougeries, Switzerland*Corresponding author: Patrick Micheels, Private office: 8, Chemin de la Fontaine, 1224 Chêne- Bougeries, Switzerland, Tel: + 41 22 347 11 13; E-mail: patrickscab@bluewin.ch
Introduction: Hyaluronic Acid (HA) gels are a commonly used option for correcting facial volume loss. Over the past 2 years, we have been testing the clinical results of two HA gels, without lidocaine, made by the same manufacturer using the patented “Inter- Penetrating NetworkLike” (IPN-Like) cross-linking technology. The aim of this work was to evaluate if there is a difference in the tissue stability and associated clinical outcomes between these two gels.
Material and methods: A 67 and 75 years old Caucasian women, from our private aesthetic center, agreed to be injected with the different HA volumiser gel, one in each of their temporal fossae. The Stylage® XL and XXL HA gels (LABORATOIRES VIVACY, Archamps, France) were used for this study. The gels have different concentrations and levels of cross-linking, but have the same indications for correcting facial volume loss resulting from the ageing process. Clinical outcomes included assessing the degree of pain experienced by participants during and after injection using a Visual Analog Scale (VAS). Aesthetic improvement was evaluated using the Merz Analogic Scale® (MAS)® and Global Aesthetic Improvement Scale (GAIS) scales completed by patients and independent experts at different pre-set time-points using photographs taken from the front, profile and three-quarter left and right angles. Moreover, Serial magnetic Resonance Imaging (MRI) scans were used to assess the tissue behavior of the gels immediately after injection and then 6 monthly for 2 years.
Results: Both gels led to an improvement in the patients’ clinical outcomes. This improvement persisted over time. The MRI scans showed that, at Day 0, the injected gel was spherical in shape and became elongated by 6 months. This appearance did not change throughout the follow-up period. The scans showed little resorption at 24 months.
Conclusion: Despite the relatively advanced age of the two patients and the small quantities of gel injected, the IPN-Like cross-linked hyaluronic acid volumiser gels had a satisfactory sustainable clinical effect as assessed by patients and experts. There was a slight advantage in favor of the gel with the lower HA concentration but higher level of cross-linking (Stylage® XXL). Given the observed persistent effect of the gels in our preliminary observation, it is now important to conduct a larger study with longer follow- up.
Hyaluronic acid; Volumiser gels; MRI; Isovolumetric resorption; Temporal fossa; IPN-Like; Cross linked
Skeletonisation of the face happens as a result of the ageing process. The same goes for the hands, which are too often overlooked [1]. Facial volume loss is becoming an increasingly important issue, as people are living increasingly longer. Indeed, in our practice, management of facial volume loss has superseded wrinkles filling in facial treatments. One of the important facial areas that suffer volume loss is the temporal fossa.
Hyaluronic Acid (HA) is a polysaccharide and an extracellular matrix key molecule. It plays an important role in maintaining tissue structure and vascularity [2,3]. HA has a wide range of activities including the stimulation of fibroblast anabolism, interfering with the intercellular communication and transport of nutritive elements between cells. It has been demonstrated that the stretching of the fibroblasts, by injecting the HA filler, stimulates the metabolism of those cells [4-7].
HA bio-implants have been successfully used for volume loss correction in several medical disciplines. Stylage® XL and XXL (LABORATOIRES VIVACY, France) are sterile dermal fillers, from non-animal origin, manufactured using the patented “InterPenetrating Network-Like” (IPN-Like) cross-linking technology. Both products constitute of HA gel together with mannitol but with varying concentrations of 26 and 21mg/g respectively and designed for use as dermal fillers in cases of facial volume restoration. The aim of these preliminary observations was to evaluate if there is a difference in the tissue stability and associated clinical outcomes between these two gels.
Two Caucasian women aged 67 and 75 years from our private aesthetic patients agreed to participate in the study. Both participants were assessed for any contraindications to theuse of HA fillers including personal or family history auto-immune disorders, granulomatous disease, active sinusitis or dental infections. Both women were provided with written and oral explanation of the procedure and study and given a 15-day cooling- off period prior to consenting to participate. The observations were conducted in full compliance with the Helsinki declaration. Participants also had full rights for their images and were fully aware that they would not be used for any purpose without their prior full consent. The volumiser product was used within the recommended manufacturer and licensing indications and contraindications.
The two HA volumiser gels used were Stylage® XL (26 mg/ml HA, batch no.: EKI18075F 2020-08) and Stylage® XXL (21 mg/ml HA, batch no.: EDF18012H 2020- 06). Both gels are manufactured by LABORATOIRES VIVACY using IPN-Like cross- linking technology and with a cohesiveness rated between 4.5-4.9 out of 5 according to Sundaram H, et al. [8]. We chose gels not containing lidocaine to avoid its potential impact on the rheological characteristics of the gel [9]. Not in any particular order, each participant was injected with the two types of gel on in either temporal fossa. Women were blind to which type of gel was being injected on either side. The HA gels were injected slowly as a bolus; using the included 27G1/2 needle (0.4 × 13 mm) (TSK Lab, Japan). The gel was injected in the antero-superior part of the temporal fossa, 1 cm above and 1 cm away from the temporal crest (Figure 1), after palpation for the temporal artery, into the deep intramuscular region, next to the bone as there is possibly no periosteum in this area [10-12]. If required, participants were given a 2nd or 3rd injections. This was then followed by a light manual massage to smooth out the gels.

Figure 1: Site of HA injection over the temporal fossa.
The behavior of the gel and its residual volume overtime were evaluated using MRI scans performed at baseline before the injections and immediately After Treatment (AT). Follow-up MRIs were then done at 6 (M6), 12 (M12), 18 (M18) and 24 (M24) months. Scans were performed at the radiology institute MedImage, Geneva, Switzerland. We used an Achievia 1.5 Tesla MRI scanner (Philips SA Health Systems, Gland Switzerland) with 3DFLAIR, 3DT1, 3DT2 sequences. The gel’s residual volume was calculated using a tool integrated in the Philips IntelliSpace Portal 7.0 image post-processing software, which can display and measure volumetric data from 2D images. An extrapolation from 2D slices was carried out and the provided volumetric measurements were expressed in mm3 (Figures 2 and 3).
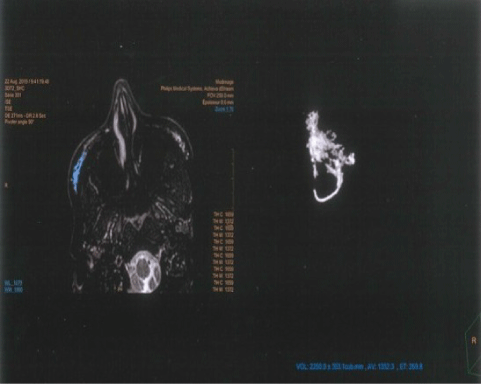
Figure 2: Example of the MRI image obtained when calculating the gel residual volume.
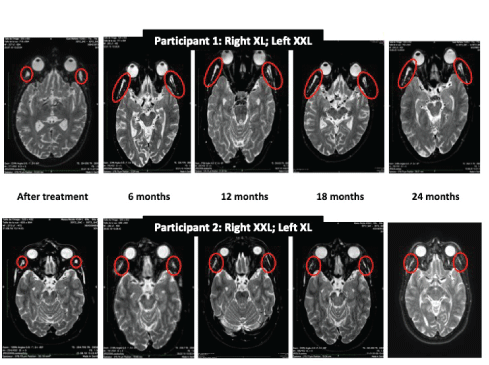
Figure 3: Sample of MRI images used to follow-up the gels’ tissue behavior.
Frontal, profile and three-quarter right and left photographs were taken before each treatment, immediately after, and then at 6 monthly intervals for 2 years. Photographs were taken using a digital camera (Nikon® DX, lens AF-S DX Nikkor, ED 18-55mm 1: 3.5-5.6 GII). These photographs were used for assessing aesthetic outcomes during the follow-up period (Figures 4 and 5).
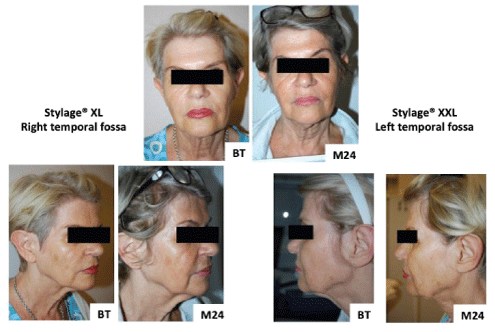
Figure 4: Participant 1 digital photographs Before Treatment (BT) and at 2 years (M24).
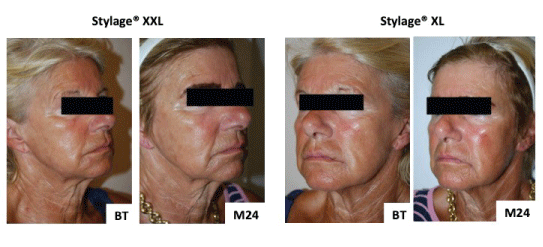
Figure 5: Participant 1 digital photographs before treatment (BT) and at 2 years (M24).
Participants were asked to quantify their pain experience at the time of injection then after 5 minutes, 30 minutes and at home using a 0-10 Visual Analog Scale (VAS) where 0 and 10 were no and unbearable pain respectively (Figure 6). Participants, the treating physician and 3 independent experts assessed aesthetic outcomes. The two participants and the independent experts were blind to which type of Stylage® was used for filling either of the temporal fossae. The participants and the treating physician compared the patient actual appearance, in the mirror or directly respectively, to their pre-treatment photo. The independent assessors used the photographs taken at the different time points to make the same comparison. Aesthetic outcomes were evaluated immediately after treatment then at 6, 12, 18 and 24 months using the global aesthetic improvement scale (GAIS) [13] and the Merz-Pharma MAS© scale [14].
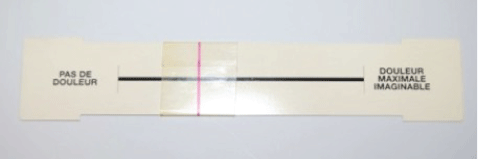
Figure 6: Pain visual analogue scale.
The study participants were aged 75 (participant 1) and 65 (participant 2) years. Their skin type and ageing were graded as III according to the Fitzpatrick classification [15] and the Glogau scale [16] respectively. The type of gel, injection side and the participants’ rated VAS pain score for each injection site at the different pre-set time points are presented in table 1.
| Right Temple | Left Temple | |||
| Participant 1 | Gel Type (Volume) | Stylage®XL, (1.0 ml) | Stylage®XXL (0.95 ml) | |
| Pain Scores | T0 | 2/10** | 1/10* | |
| T+5’ | <1/10 | 0/10 | ||
| T+30’ | 0/10 | 0/10 | ||
| At home | 1/10 | 0/10 | ||
| Participant 2 | Gel Type (Volume) | Stylage®XXL (0.50 ml) | Stylage®XL(0.50 ml) | |
| Pain Scores | T0 | 2/10** | 1/10* | |
| T+5’ | 1/10* | 1/10* | ||
| T+30’ | 0/10 | 0/10 | ||
| At home | 0/10 | 0/10 | ||
Table 1: Pain scores for different gel types at different time points.
T0: During injection; T+5’: 5 minutes after injection; T+30’: 30 minutes after injection;
* Heaviness or pressure; ** Painful discomfort.
The MRI characterizations of gel volume and behavior at different time-points are presented in figure 7 and table 2.
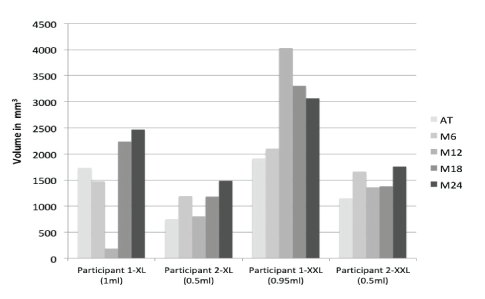
Figure 7: Gel volumes assessed by MRI at different follow-up time-points.
| Right Temple | Left Temple | ||
| Participant 1 | Gel | Stylage®XL, (1.0 ml) | Stylage®XXL (0.95 ml) |
| AT | Gel not touching the bone, located in the pre-aponeurotic plane as a linear uniform dense papule with convex, smooth edges and little infiltration between fibres (1.732). | Gel located in the pre & post aponeurotic planes in the shape of a small and very linear uniform papule with smooth convex edges (1.909 mm3). | |
| M6 | Gel has spread into the pre > post- aponeurotic plane, flattening out significantly, linear appearance with smooth. |
Gel has a linear appearance located equidistant between the pre- and post- aponeurotic regions. The structure remained. |
|
| M12 | Appearance remained stable (3.187 mm3). | Appearance remained stable (4.023 mm3). | |
| M18 | Appearance remained stable (2.236 mm3). | Appearance remained stable (3.308 mm3). | |
| M24 | Appearance remained stable (2.467 mm3). | Appearance remained stable (3.069 mm3). | |
| Participant 2 | Gel | Stylage®XXL (0.50 ml) | Stylage®XL(0.50 ml) |
| AT | Gel not touching the bone located more markedly sub- aponeurotic than super-aponeurotic as a linear uniform dense papule with convex edges (1.154 mm3). | Gel infiltrated underneath the aponeurosis not touching the bone with a dense uniform structure, concave edges and no papule (0.751 mm3). | |
| M6 | Gel extended antero-posteriorly flattening out and infiltrating caudally (1.664). | Gel became narrower and extended caudally (1.191 mm3).* | |
| M12 | Appearance remained stable (1.571 mm3). | Images show no more papules but remained visible extending distally. |
|
| M18 | Appearance remained stable (1.384 mm3). | Papule not visible in the temporal fossa (1.180 mm3). | |
| M24 | Appearance remained stable (1.757 mm3). | Volume is 1,484 mm3, or a little less than 3 times the injected volume. |
Table 2: MRI characterization of gel behavior and estimated volume in the temporal fossae at different time-points.
*The images were not optimal because of movement but still able to be interpreted by the radiologist and by the machine.
With regards to the aesthetic outcomes as assessed by the MAS® scale, participant 1 reported a 2 point improvement compared to baseline for both the right and left sides immediately following treatment. This improvement persisted throughout the follow-up period. Participant 2, despite the smaller amount of gels injected, reported also a two point improvement on the MAS® scale for each side immediately after treatment. This level of improvement increased to three points at 12 months in the Stylage® XXL and then returned back to 2 points, while on the other side the patient considered that her improvement increased (3 points compared to baseline) at 6 months and this level of improvement was sustained throughout the rest of the follow-up (Table 3). There was variation in the experts individual MAS® scores of both participants throughout the followup (Table 4), yet their average score still demonstrated improvement in both participants. This improvement was marginally better and more sustainable in participant 2 for the side injected with Stylage® XL compared to XXL (Table 3).
| Right temple | Left Temple | ||||||||||||
| Participant 1 | Gel | Stylage®XL, (1.0 ml) | Stylage®XXL (0.95 ml) | ||||||||||
| Assessor | Participant’srating | Average expert’ rating | Participant’srating | Average expert’ rating | |||||||||
| Scale | MAS score |
ΔMAS | GAIS | MAS score |
ΔMAS | GAIS | MAS score |
ΔMAS | GAIS | MAS score |
ΔMAS | GAIS | |
| BT | 3 | - | - | 3 | - | - | 3 | - | - | 3.5 | - | - | |
| AT | 1 | 2 | 4 | 1.5 | 1.5 | 3.25 | 1 | 2 | 4 | 1.5 | 2 | 3.5 | |
| M6 | 1 | 2 | 4 | 1.75 | 1.25 | 3 | 1 | 2 | 3 | 1.25 | 1.75 | 3.25 | |
| M12 | 1 | 2 | 4 | 2.25 | 0.75 | 2 | 1 | 2 | 4 | 1.5 | 2 | 3 | |
| M18 | 1 | 2 | 3 | 2 | 1 | 2.25 | 1 | 2 | 4 | 1.75 | 1.75 | 3 | |
| M24 | 1 | 2 | 3 | 1.5 | 1.5 | 2.5 | 1 | 2 | 4 | 1.75 | 1.75 | 2.75 | |
| Participant 2 | Gel | Stylage®XXL (0.50 ml) | Stylage®XL(0.50 ml) | ||||||||||
| Assessor | Participant’sscore | Average expert’ rating | Participant’sscore | Average expert’ rating | |||||||||
| Scale | MAS score |
ΔMAS | GAIS | MAS score |
ΔMAS | GAIS | MAS score |
ΔMAS | GAIS | MAS score |
ΔMAS | GAIS | |
| BT | 3 | - | - | 3 | - | 4 | - | - | 3 | - | |||
| AT | 1 | 2 | 4 | 1.5 | 1.5 | 2.75 | 2 | 2 | 4 | 1.5 | 1.5 | 2.75 | |
| M6 | 1 | 2 | 4 | 1 | 2 | 2.75 | 1 | 3 | 4 | 1 | 2 | 2.5 | |
| M12 | 0 | 3 | 3 | 1.25 | 1.75 | 2.5 | 1 | 3 | 3 | 0.75 | 2.25 | 2.5 | |
| M18 | 1 | 2 | 4 | 1.5 | 1.5 | 2.75 | 1 | 3 | 4 | 1.5 | 1.5 | 2.5 | |
| M24 | 1 | 2 | 3 | 1.25 | 1.75 | 2 | 1 | 3 | 3 | 1.25 | 1.75 | 2.25 | |
Table 3: MAS© and GAIS scores as evaluated by participants and experts at the different time points.
ΔMAS: Points of improvement compared to before treatment; BT: Before treatment; AT: After treatment; M6, M12, M18 and M24: 6, 12, 18 and 24 month follow-up time points.
| Expert | BT | AT | M6 | M12 | M18 | M24 | |||||||
| Participant 1 | R | L | R X |
L XX |
R X |
L XX |
R X |
L XX |
R X |
L XX |
R X |
L XX |
|
| 1 | 3 | 4 | 2 | 2 | 1 | 1 | 3 | 2 | 2 | 2 | 1 | 2 | |
| 2 | 4 | 4 | 2 | 2 | 3 | 2 | 3 | 2 | 3 | 3 | 2 | 3 | |
| 3 | 2 | 3 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | |
| 4* | 3 | 3 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | |
| Participant 2 | R | L | R XX |
L X |
R XX |
L X |
R XX |
L X |
R XX |
L X |
R XX |
L XL- |
|
| 1 | 3 | 4 | 1 | 2 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 0 | |
| 2 | 4 | 4 | 3 | 3 | 1 | 2 | 2 | 2 | 2 | 2 | 1 | 1 | |
| 3 | 3 | 2 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | |
| 4* | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | |
Table 4: Evaluation of the efficacy of treatment according to the MAS® scale by experts.
*Physician who performed the injections. XL: Stylage® XL; XXL: Stylage® XXL; R: Right; L: Left; BT: Before treatment; AT: After treatment; M6, M12, M18 and M24: 6, 12, 18 and 24 month follow-up time points.
Based on participants and experts ratings using GAIS, there was also evidence of aesthetic improvement. This was slightly better in the side injected with Stylage® XXL compared to XL, particularly in participant 1 (Tables 3 and 5).
| Expert | AT | M6 | M12 | M18 | M24 | ||||||
| Participant 1 | R X |
L XX |
R X |
L XX |
R X |
L XX |
R X |
L XX |
R X |
L XX |
|
| 1 | 3 | 3 | 4 | 3 | 2 | 3 | 3 | 3 | 3 | 2 | |
| 2 | 4 | 4 | 3 | 3 | 1 | 2 | 1 | 2 | 2 | 2 | |
| 3 | 2 | 3 | 2 | 3 | 2 | 3 | 2 | 3 | 2 | 3 | |
| 4* | 4 | 4 | 3 | 4 | 3 | 4 | 3 | 4 | 3 | 4 | |
| Participant 2 | R XX |
L X |
R XX |
L X |
R XX |
L X |
R XX |
L X |
R XX |
L XL- |
|
| 1 | 4 | 4 | 3 | 3 | 4 | 4 | 4 | 4 | 3 | 4 | |
| 2 | 3 | 3 | 3 | 3 | 1 | 1 | 1 | 2 | 1 | 3 | |
| 3 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | |
| 4* | 4 | 4 | 4 | 4 | 4 | 4 | 3 | 3 | 3 | 3 | |
Table 5: Evaluation of improvement in the temporal fossa according to the GAIS scale, by experts.
*Physician who performed the injections. XL: Stylage® XL; XXL: Stylage® XXL; R: Right; L: Left; AT: After treatment; M6, M12, M18 and M24: 6, 12, 18 and 24 month follow-up time points.
There were no reported or observed adverse events apart from a small hematoma at the injection site in relation to the right temporal fossa in participant one.
In this report on 2 women over a 2-year follow-up period we were able to monitor the behavior of two cross-linked HA gels developed using the patented IPN-Like technology. Using multiple measures, we were able to demonstrate that the aesthetic effect of both types of gels was comparable, as assessed by women and experts. This effect was also sustained throughout the follow-up period. The persistence of the effect of different types of volumiser gels had been previously demonstrated in several other studies [17-20]. However, an interesting finding in our observations is how fast the volume of the HA gel increased soon after injection. Indeed, as measured on MRI, approximately 30 minutes after injection, the gel volume was higher than the volume injected. This volume continued to increase over time reaching almost 3 times the initially injected volume by 24 months.
Exploring the mechanism behind this rapid volume expansion is beyond the scope of this report; hence our proposed reasons are only speculative. This increase in volume could simply be a result of the HA’s hydrophilic characteristics. It is also plausible that this could be due to a phenomenon similar to that previously described by Q-Med laboratory in relation to the isovolumetric resorption of NASHA gels. We believe that it is unlikely that this observation was secondary to a mere post-trauma oedema because of its persistence beyond 3 weeks. However, it is possible that this could be a reactive oedema, without cellular response, or secondary to a reduction in lymph drainage [21]. Nevertheless, there were some fluctuations in both the measured volume and the subjective assessments, which could have been caused by the day-to-day variations in participants’ perception of their own body images or dietary intake.
The injection of both gels was well tolerated by both of our participants despite that both gels did not have lidocaine. We did not use lidocaine containing gels to avoid its potential effect on altering the rheological characteristics of the gel [9]. We performed the injections using a needle, inserted perpendicular to the skin surface, into the temporal fossa. This type of injecting is fairly painless and easy to perform. We also aimed to getin contact with the bone to reduce the risk of intravenous injection and embolism. Furthermore, this technique is very effective in the antero-superior part of the temporal fossa, where the muscle is very thin. Nonetheless, it should be emphasized that this technique does not abolish this risk altogether. Even though the needle comes into contact with the bone, the gel is not placed directly against the bone as the tip of the needle is bevelled. Moreover, the gel does not separate the muscle fibres from their attachment to the bone. Following injection, the gel seemed to become distributed in bulk from the point of injection as well as along the muscle fibres. We believe that the presence of gel above the aponeurosis could probably be either the result of its migration along the needle track or due to a slight retrograde injection [22].
We recognize that the small number of participants is a limitation to our observations and hence our findings should be interpreted with caution. However, the duration of the follow-up, the use of multiple, objective and subjective, validated measures and the masking of most of the assessors in this study are strengths to our design.
Over a period of 2 years, we monitored the behavior and resorption levels of two hyaluronic acid volumiser gels that use the patented “Inter-Penetrating Network-Like” cross-linking technology, with different concentrations and levels of cross-linking. We monitored the patients under clinical conditions using photographs and MRI. We requested the blind opinions of the patients and 3 experts. Although cross-linked hyaluronic acid gels are said to have a half-life of 6-24 months, we demonstrated that, at least for the 2 volumiser gels we experimented with, they had a longer persistence and clinical efficacy in the temporal fossa. It is important that our findings are confirmed in larger studies.
The authors would like to sincerely thank the two study participants for their involvement. We also want to thank Dr O Jordan, MDPhD, for providing the expert advices, Dr D Sarazin, Institut Viollier, Geneva (Switzerland), for their help with researching the anatomy of the periosteum in the temporal fossa, Mrs Maria Ferros, secretary to P Micheels, for her valuable work, laboratoire Vivacy-Archamps, France for supplying the HA gels used for this study and The radiology institute MedImage, Geneva, Switzerland, for allowing us to use their equipment. We would also like to acknowledge Superviseme LTDmedical writing services- (http://www.superviseme.eu) for their support with the medical writing and editing of the manuscript, which was funded by LABORATOIRES VIVACY.
The syringes were donated by the LABORATOIRE VIVACY, Archamps, France. Dr St Besse was remunerated for the work she performed. Drs P Micheels, B Elias, J Vandeputte declare no conflict of interest, having received no financial compensation in return for the work carried out. Dr Micheels is a consultant and trainer for Allergan, IBSA, Galderma Q-Med Switzerland, KioMed, Kylane, Merz, Sinclair, Teoxane and Vivacy. Dr Vandeputte is a consultant and trainer for Merz and Advanced Aesthetic Technologies.
Download Provisional PDF Here
Aritcle Type: RESEARCH ARTICLE
Citation: Micheels P, Besse S, Elias B, Vandeputte J (2022) Stability of Two Cross-Linked Hyaluronic Acid Gels for the Treatment of Temporal Fossa Volume Loss: A Preliminary Study Using MRI and Visual Observations. J Clin Cosmet Dermatol 6(2): dx.doi.org/10.16966/2576-2826.172
Copyright: © 2022 Micheels P, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access