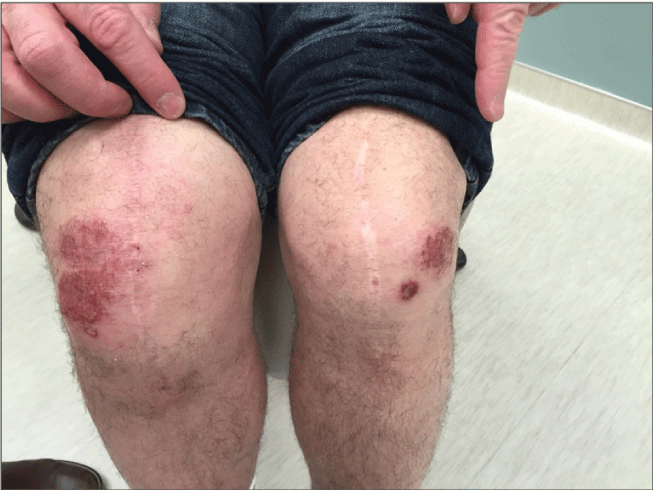
Figure 1: A photograph of the patient’s rash which developed 3 months post-operatively. Skin biopsy demonstrated epidermal acanthosis with prominent spongiosis, suggesting an allergic contact dermatitis.


Darren D Lynn1* Drew H Smith2 Jeffrey A Arthur3 Cory A Dunnick4,5
1University of Colorado School of Medicine, Aurora, Colorado, USA*Corresponding author: Darren D Lynn, University of Colorado School of Medicine, Aurora, Colorado, USA, Tel: (208) 546-2284; E-mail: Darren.lynn@ucdenver.edu
Contact allergy to metals in total knee arthroplasties is a well-documented event due to its occurrence in the general population; however, sensitivity to other components, such as the chemicals used in the bone cement, is becoming more prominent as joint replacement surgeries continue to rise. We report a case of benzoyl peroxide (BPO) allergy leading to a persistent skin reaction in a patient with normal mechanical function after bilateral total knee arthroplasty. A thorough review of the literature reveals all published cases of orthopedic implant patients with dermatological reactions and sensitivity to benzoyl peroxide concomitantly showed symptoms of joint failure, including pain, swelling, and prosthetic loosening. Our case study presents a patient who has developed a delayed-type sensitivity reaction without joint dysfunction of any kind. This clinical presentation is a variant of those previously reported and represents a new genre of treatment challenges for surgeons and dermatologists. In this article, we discuss the implications this case has in preventing post-surgical complications and possible treatment options for this novel patient presentation.
Benzoyl Peroxide; Dermatitis; Replacement; Allergy; Patch testing
In 2010, the National Hospital Discharge data survey reported that 719,000 total knee replacements (TKA) were performed in the United States [1]. Persistent sensitivity reactions to components of the prostheses can also occur and, at times, contact allergens can lead to early joint failure. Contact allergies have been reported to metal components including nickel, cobalt, and chrome as well as components of bone cement including acrylates, benzoyl peroxide and added antibiotics [2]. The cases of hypersensitivity towards the latter category are not new among orthopedic implant patients, however, knowledge of these types of reactions is an important preoperative consideration in selecting patient hardware and accessories [3].We report a case of benzoyl peroxide allergy leading to persistent skin reactions in a patient with normal mechanical function after bilateral total knee arthroplasty. Prior published case reports of hypersensitivity to benzoyl peroxide have involved patients with aseptic loosening and swelling of the joint thus obscuring whether or not the sensitization was primary or secondary to the problems of the implanted prosthesis [4]. Our patient’s ongoing skin symptoms are not accompanied any reported joint problems consistent with previous reports, presenting a new possible treatment challenge to future TKA patients. It is important for physicians addressing patients’ joint replacement-linked skin reactions to consider the novel clinical presentation described to generate an adequate treatment plan. In this case report, we discuss the implications this case may have for surgeons performing prosthetic implant procedures and possible treatment options for this novel patient presentation.
A 61-year-old male with a past history of a complete left knee replacement (April 2013) was referred for patch testing after a 6-month history of bilateral knee dermatitis following a recent right knee replacement (April 2014). The surgical history of the most recent knee replacement revealed an immediate post-operative skin reaction to Derma-bond (containing ethyl cyanoacrylate) which resolved, however, developed a new skin reaction unrelated to topical products 3 months later (Figure 1). Surgical notes indicated a Striker prosthesis composed of a nickel-cobalt-chrome alloy with Simplex P bone cement was used to secure the joint while tobramycin was added as a prophylactic disinfectant. Skin biopsy on presentation to the dermatology clinic showed epidermal acanthosis with prominent spongiosis and primarily lymphocytic infiltrate, suggestive of an allergic contact dermatitis (histology not included). The patient’s rash was controlled with a topical clobetasol applied regularly. The contact dermatitis cleared a week within the application of the corticosteroid and did not progress to any other locations throughout this time. The patient stated dermatitis would return within days of not using a topical steroid. Notably, the patient denies joint pain or swelling and demonstrates the good function of both knees since his knee replacement 29 months ago (Figure 2).

Figure 1: A photograph of the patient’s rash which developed 3 months post-operatively. Skin biopsy demonstrated epidermal acanthosis with prominent spongiosis, suggesting an allergic contact dermatitis.
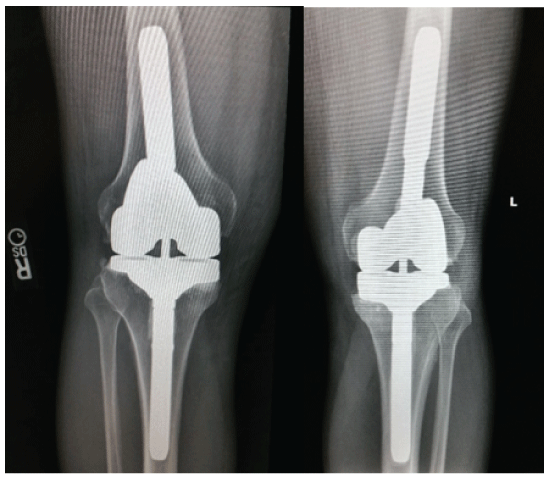
Figure 2: Radiographs of patient’s knee replacements. From left to right: right leg, left leg
Patch testing to a North American standard and metal series revealed reactions to ethyl cyanoacrylate (+1) and benzoyl peroxide (+1) (Figures 3A and 3B). Positive reaction to ethyl cyanoacrylate explained his prior skin reaction to Derma-bond. Benzoyl peroxide positivity was thought to be relevant to the Simplex P bone cement which uses benzoyl peroxide as an initiator. Additional reactions to formaldehyde (+1) and methyl dibromo glutaronitrile (+1) were of doubtful relevance to his reaction given their absence in knee replacement products and surgeries. The patient had no reactions to methyl methacrylates, metals and neomycin despite reporting a history of neomycin allergy
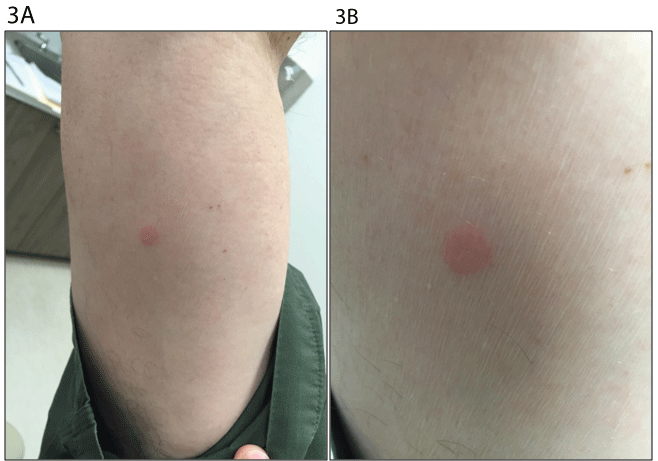
Figure 3: Photographs of the results to the North American standard and the metal series patch testing revealing reactions to ethyl cyanoacrylate (+1) and benzoyl peroxide (+1).
As our population ages, total knee arthroplasties will continue to become more common, with an anticipated million surgeries done in 2030 [5]. The incidence of post-surgical complications from an allergy to components of joint prostheses would also be expected to rise accordingly. Contact allergy to metals is well-documented because of its prevalence in the general population, with nickel allergy occurring in more than 3% of men and 17% of women in the United States [6]. Sensitivity to other components used in bone cement is also becoming more prominent; one study (n=239) reported delayed-type hypersensitivity reactions to bone cement components in 24.8% of patients, with benzoyl peroxide (BPO) having some of the highest prevalence [5].
Simplex P is one of the most commonly used bone cements and comes in two parts: powder and liquid, with methacrylate (MMA) being a common constituent in both [2]. The powder consists of MMA beads, serving as the pre-polymerized poly, and BPO, serving as the initiator and a radio pacifier [7]. The liquid portion consists of MMA and N,Ndimethyl-para-toluidine (DMPT)—an activator [2]. When the liquid and powdered portions are mixed at room temperature, BPO and DMPT produce free radicals and initiate the exothermic polymerization reaction of MMA until the cement begins to harden [8]. Benzoyl peroxide, when pushed into the injured blood vessels in bone during implantation, is thought to act as a hapten that conjugates with proteins in the patient’s body, creating a neoantigen capable of initiating an immune response [9]. The resultant delayed-type sensitization reaction manifests on the skin as eczema or dermatitis [3].
In the general population, patch test interpretation with BPO should be approached with caution, as it is a known irritant and can cause a reaction in up to 7.8% of individuals with a 1% solution [10]. Rates among patients with past dental procedures, acne product use, and history of joint replacement, however, have a higher prevalence of positive reactions that range from mild (+1) to severe (+3) [4,11]. Given our patient’s lack of past exposure to BPO-containing products or dental procedures and negative patch results to all other knee replacement hardware or bone cement, we are confident his dermatitis is a result of a delayed-type hypersensitivity reaction. We hypothesize that our patient became sensitized to BPO after his first knee replacement in April 2013 and developed a dermatitis rash on both knees when re-exposed after his second knee replacement in April 2014.
Interestingly, all published cases of orthopedic implant patients presenting with a rash and positive patch sensitivity to BPO began to show dermatitis around 4-8 weeks after their surgery—much like our patient. However, unlike our report, these patients concomitantly had signs and symptoms of a joint failure, obscuring whether the sensitization was primary or secondary to a damaged joint [4,12-14]. Our patient still has not shown signs of joint dysfunction, suggesting a delayed-type hypersensitivity reaction precedes major joint complications.
In most cases, removal of the offending device clears dermatitis; however, screening before the surgery for potential allergens through patch testing would be an excellent precautionary method. To illustrate its potential, one report documents this precaution on a 60-year-old woman with an indicated right total knee arthroplasty and history of allergic reactions to artificial acrylic-based fingernails and temporary dental fillings [15]. Patch testing was performed with a panel of bone-cement components confirming an MMA allergy [15]. The use of a cemented knee arthroplasty was contraindicated and the patient underwent a right total knee arthroplasty using non-cemented, porous in growth components [15]. The patient’s post-operative course was uneventful and was reportedly walking unlimited distances without assistive devices two years later [15].
We recommend a complete and thorough patient history and skin exam to identify possible allergens and distinguish between allergic and contact dermatitis. Systematic patch test screening for contact allergy related to a joint prosthesis, a panel of metals, acrylates, cement components, antibiotics and skin preps should be considered (Table 1). For patients sensitive to BPO or other bone cement chemicals, we suggest a cementfree prosthesis, which can have a similar life span to cemented prostheses [16].
Although contact allergy to joint components is not common, it should be considered especially in cases of aseptic joint inflammation with or without surrounding skin involvement. In cases such as these, barring no progression of prosthesis involvement, we suggest observation of the joint function. Intra-articular steroid injections as a prophylactic measure are ill-advised as this would introduce an increased risk of infection. Rather, it is suggested that intra-articular steroid injections be saved for symptomatic relief only
Download Provisional PDF Here
Aritcle Type: Case Report
Citation: Lynn DD, Smith DH, Arthur JA, Dunnick CA (2017) Successful bilateral total knee replacements with ongoing allergic contact dermatitis to benzoyl peroxide. J Clin Cosmet Dermatol 1(1): doi http://dx.doi.org/10.16966/2576-2826.107
Copyright: © 2017 Lynn DD, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access