Abstract
Although aortic aneurysms associated with Takayasu arteritis are not rare, a total occlusion of the thoracoabdominal aorta with enlargement is rare. We have experienced a 60-year-old man who underwent a second-stage surgery for thoracoabdominal aortic aneurysm nine years after the prior operation of an ascending aorta-abdominal aorta bypass for atypical coarctation due to Takayasu arteritis. We present a successful case of the treatment result after 4 years’ follow-up.
Keywords
Atypical Coarctation; Thoracoabdominal Aortic Aneurysm; Ascending Aorta-Abdominal Aorta Bypass; Takayasu Arteritis; A secondstage surgery
Introduction
Although aortic aneurysms associated with Takayasu Arteritis (TA) are not rare, a total occlusion of the thoracoabdominal aorta with enlargement is rare [1,2]. We experienced a case who underwent surgery of the graft replacement for the enlarged Thoracoabdominal Aortic Aneurysm (TAAA) 9 years after the prior operation of an ascending aorta -abdominal aorta bypass for atypical coarctation due to TA.
Case Report
A 60-year-old male was admitted to our hospital because of an enlarged TAAA after the ascending aorta-abdominal aorta bypass for atypical coarctation due to TA [3]. At the age of 51 years old, he was admitted to our hospital because of the upper extremity hypertension and vascular claudication of the lower extremities (Figure 1). He underwent the extra anatomical ascending aorta-abdominal aorta bypass with a 16 mm prosthetic graft and reconstruction of bilateral renal arteries and Superior Mesenteric Artery (SMA) with Saphenous Vein Grafts (SVGs) and reattachment of the Inferior Mesenteric Artery (IMA) directly to the aortic graft under a midline sterno-laparotomy incision without cardiopulmonary bypass (Figure 2A). After surgery, upper extremity hypertension and vascular claudication were completely resolved. The etiology of atypical coarctation had related to TA because of the angiographic findings and positive specific alleles such as HLA-B⁎ 5201 and DRB1⁎ 1502 [4,5]. The patient had been doing well without antihypertensive drugs for nine years since his operation. The diameter of TAAA dilated from 46 mm to 70 mm in nine years after the prior operation (Figure 2B). Routine laboratory work, including C-reactive protein and erythrocyte sedimentation rate, was normal. Because of the risk of rupture, surgical intervention was indicated. Preoperative spinal cord Computed Tomography Angiography (CTA) showed that Adamkiewicz Artery (AKA) was arising from a left posterior lumbar artery at the level of the second lumbar artery (Figure 3).
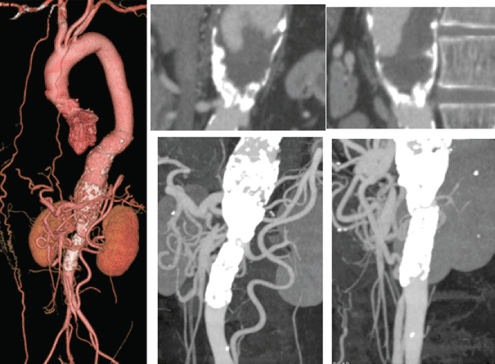
Figure 1: Preoperative Computed Tomography Angiography (CTA) of the initial operation. The descending thoracic aorta gradually dilated into the abdominal aorta. Complete occlusion of the thoracoabdominal aorta and the Superior Mesenteric Artery (SMA) and 75% stenosis of the Inferior Mesenteric Artery (IMA) were observed. The dilated IMA was a major collateral supply to the SMA.
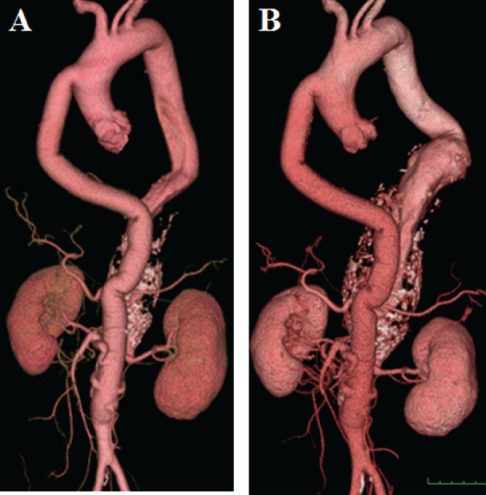
Figure 2: A: CTA immediately after the initial operation. B: CTA nine years after the initial operation. The diameter of TAAA dilated from 44mm to 68mm in nine years. Aorto (graft)-SMA bypass using Saphenous Vein Graft (SVG), aorto-bilateral renal artery bypasses using SVGs, and IMA were all patent.
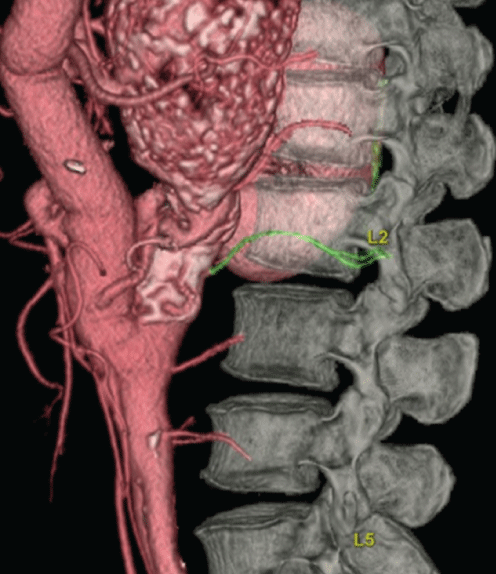
Figure 3: Preoperative spinal cord CTA. Note that Adamkiewicz artery was arising from a left posterior lumbar artery at the level of the second lumbar artery.
Under general intubation anesthesia using double lumen tube with the patient placed in a right half lateral position using intraoperative Motor Evoked Potential (MEP) monitor, a left thoracoabdominal incision was made beginning at 3 cm below scapula and continued in posterolateral thoracotomy in the seventh intercostal space resecting the eighth rib. The incision was extended oblique across the costal cartilage and continued in oblique abdominal incision. The lateral half of the diaphragm was divided circumferentially 3 cm from its origin and the left crus was detached from the spine. This permitted the diaphragm to collapse medially to expose the lower thoracic aorta. By retracting the viscera to the right, a retroperitoneal space, TAAA, and Celiac Artery (CA) were exposed (Figure 4A). TAAA was markedly expanded. The descending thoracic aorta at the takeoff level of the seventh Intercostal Artery (ICA) was exposed. After systemic heparinization, the thoracic aorta proximal to the TAAA was double-clamped and the aorta was transected. The proximal anastomosis between the transected proximal aorta and 22 × 11mm bifurcated J graft (J Graft SHIELD NEO, Japan Lifeline, Tokyo, Japan) was performed with continuous 3-0 polypropylene sutures using an outer Teflon felt strip. The CA was clamped. The TAAA was opened. Thrombotic materials were removed from within the aneurysm. A small amount of back-bleeding from the 7th, 9th and 11th ICAs was observed. The back-bleeding from the11th ICA was controlled with a 2-French gauge Fogarty balloon catheter which was inserted from within the opened aorta. The other ICAs were over sewn. The orifice of CA was found within the aneurysm. The CA was cannulated with a 14-French gauge Foley balloon catheter and perfused with cold saline solution. The distal anastomosis between the right limb of the graft and the CA was made with 4-0 polypropylene continuous sutures using an outer Teflon felt strip. Air was evacuated from the graft and circulation to the CA was restored. The distal anastomosis between the left limb of the graft and the 11th ICA was made with 4-0 polypropylene continuous sutures. Air was evacuated from the graft and circulation to the 11th ICA was restored (Figure 4B). Protamine sulfate was given. The wound was rinsed with saline. The aneurysmal wall was sutured over the graft. Drainage tubes were placed. After reapproximating the diaphragm, the costal cartilage was repaired. The chest was closed. During operation, hemodynamic state was stable and the MEP monitor was not changed. The operating time was 4 hours and the total blood loss approximately 2000ml. On the day of operation, he received 840 ml of blood, 720 ml of fresh-frozen plasma and 800 ml of platelets.
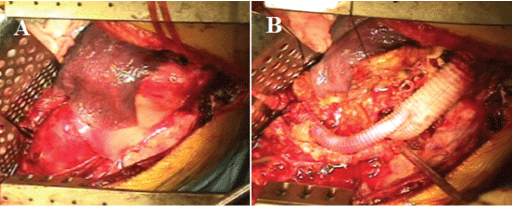
Figure 4: Operative findings. A: The aneurysm was exposed. B: The graft replacement was performed.
The patient was extubated the next day, Cerebrospinal Fluid Drainage (CFSD) was discontinued on Postoperative Day (POD) 2, and paraplegia was prevented. The patient was discharged on POD18. Postoperative CTA demonstrated a patent aorto-CA bypass graft and an occluded left limb of the graft (Figure 5). Microscopic examination of tissue from the aneurysmatic aortic wall demonstrated remarked atherosclerosis with thickening of the intima, but no active inflammation in the media. In the adventitia, chronic inflammation was demonstrated. The patient is now doing well 4 years after the second-stage operation.
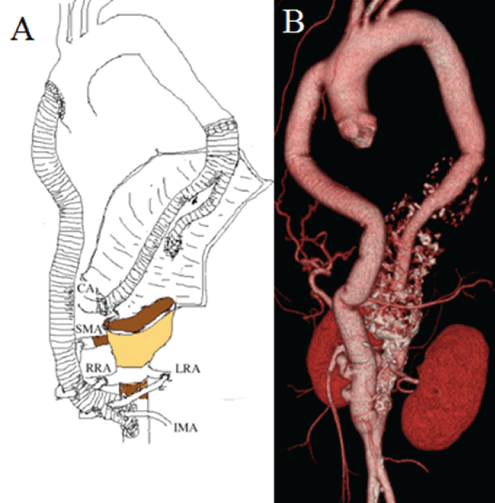
Figure 5: A: Schematic drawing of the recent operation. B: Postoperative CTA demonstrated the patent aorto-CA bypass graft and the occluded left limb of the graft. CA, Celiac Artery; SMA, Superior Mesenteric Artery; RRA, Right Renal Artery; LRA, Left Renal Artery; IMA, Inferior Mesenteric Artery.
Discussion
TA is a rare autoimmune arteritis that affects the aorta and its main branches, and may require surgical intervention for treatment of occlusive and aneurysmal lesions [1,2,6,7]. Aneurysmal formation is one of the major complications related to the prognosis in TA [1,2]. The high frequency of aortic aneurysm in association with TA has also been confirmed in surgical series: 30% according to Crawford ES, et al. [8], and 48% according to Kieffer E, et al. [2]. But a total occlusion of TAAA due to TA is rare [1,2].
Spinal cord injury such as paraparesis and paraplegia remains one of the major concerns in surgery on the TAAA [9]. For spinal cord protection, various adjuncts, such as distal aortic perfusion, hypothermia, partial CPB, preservation or reattachment of the responsible intercostal or lumbar arteries, CSFD, and pharmacologic agents, have evolved [9]. There is growing evidence that the AKA may, nowadays, be visualized through noninvasive methods, such as magnetic resonance angiography or CTA [10,11]. Preoperative detection of the AKA is useful for establishing the best operational strategy for TAAA repair [10,11]. In the present case, the AKA was arising from a left posterior lumbar artery at the level of the second lumbar artery.
In the present case, the diameter of the TAAA increased by 2.4 cm over 9 years and 0.27 cm per year. Sueyoshi E, et al. [7] reported that the growth rate of atherosclerotic aortic aneurysms was 0.34 cm/year, whereas the growth rate of aneurysms in patients with TA was 0.03 cm/year. They also showed that calcium deposits in the scarred media and intima seems to limit further enlargement of aortic aneurysms. Aortic wall scars are more severe in patients with TA than in those with atherosclerosis. Therefore, aneurysms associated with this disease increase in size more slowly than atherosclerotic aortic aneurysms. But in the present case, the TA aneurysm with calcification dilated as same as atherosclerotic aortic aneurysms because blood pressure had applied due to complete occlusion of the abdominal aorta.
In the present case, most blood in the preoperative TAAA had flowed into the CA and ICAs. Preoperative CTA findings showed that the aortic diameter on the proximal side of the TAAA was 24 mm and the diameter of CA was 9 mm. Because there was no tapered prosthetic graft having a proximal graft diameter of 24 mm and a distal graft diameter of 9 mm to our knowledge, a 20 × 10 mm bifurcated graft was used. The right limb of the graft was anastomosed to the CA and the left limb of the graft to the 11th ICA without difficulty [12]. In postoperative CTA, unfortunately the left limb of the graft had been occluded, but fortunately paraplegia was prevented. In the present case, the AKA was arising from a left posterior lumbar artery at the level of the second lumbar artery and the below of the surgical field.
Successful endovascular aneurysmal repair (stent-grafting) for dilated lesions due to TA has been reported [1]. In the present case, we evaluated the possibility of hybrid intervention with the use of endovascular stent graft, but open surgery was selected because access artery was absent.
In conclusion, reports of the second-stage surgery for the TAAA with an atypical coarctation due to TA are very rare [13,14]. After surgery for TA, anastomotic false aneurysms and graft deterioration can occur at any time in the long term [1,13-15], and so, careful follow-up is mandatory.
Informed Consent
Written informed consent was obtained from the patient for the publication of this case report.
Disclosure Statement
The present case was reported as a case report to the journal of the Japanese Society for Cardiovascular Surgery in 2013 about the first operation [3].
Conflict
All authors have no conflict of interest.
Article Information
Article Type: CASE REPORT
Citation: Harada H, Isa H, Miyamoto H, Nakastu T, Kimura F, et al. (2022) A Second-Stage Surgery for Thoracoabdominal Aortic Aneurysm after Ascending Aorta-Abdominal Aorta Bypass for Atypical Coarctation Due to Takayasu Arteritis. J Clin Case Stu 7(3): dx.doi.org/10.16966/2471- 4925.256
Copyright: © 2022 Harada H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
Received date: 17 May, 2022
Accepted date: 09 Jun, 2022
Published date: 16 Jun, 2022






