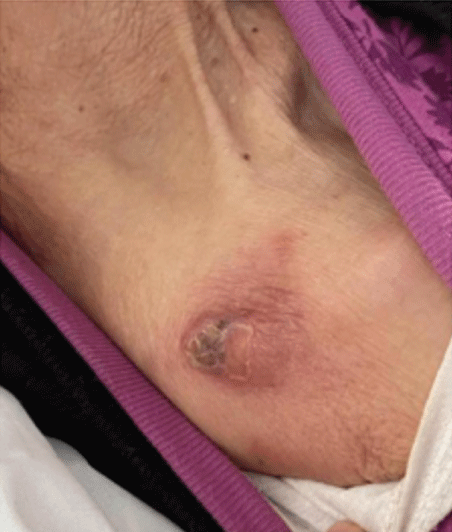
Figure 1: Awalnut-sized red mass can be seen on the right shoulder part of the lesion had rupture scab.


Wang Lijie Jiang Feng Zhao Qian Jin Hui Bai Jie Liu Yang Bo Kaiping Chen Ruijuan Liu Qin yue Wu Jingjing Zhang Ziyan Zhu Hanghang Mao Xin Song Xudong Song Xudong Luo Dan Yang Jie*
Affiliated Hospital of North China University of Science and Technology, Tangshan, 063000, China*Corresponding author: Yang Jie, Affiliated Hospital of North China University of Science and Technology, Tangshan, 063000, China, E-mail: yangjj1971@126.com
A case of extranodal NK/T cell lymphoma (nasal type) with skin lesion as the first manifestation is reported. A 82 year old female presented with fever for 10 days, and mass with ulceration on right shoulder for 4 days. Clinically, a walnut-sized red mass with local ulceration and scab can be seen on the right shoulder. Part of the lesion had rupture scab. A circular-like infiltrated dark red plaque with a diameter of about 4cm can be seen on the right chest, with yellow and white dry secretion in the center. Multiple patches of dark red spots from coins to walnuts can be seen on the trunk and limbs. Based on skin lesions, histological, immunohistochemical staining and EBER in situ hybridization, a diagnose of primary cutaneous extranodal NK/T cell lymphoma, nasal type was made.
Extranodal NK/T cell lymphoma; Nasal type; Skin lesion; Primary
A 82-year-old female was first hospitalized with fever and mass with ulceration on right shoulder. The patient developed fever with decreased appetite 10 days ago without obvious cause. During this period, the highest temperature is 39.9°C, 4 days ago, a 1.5cm diameter mass was found on the right shoulder, accompanied by redness, heat and pain, swelling. A dark red infiltrating plaque, approximately 1cm in diameter, is seen on the inside of the right breast. No cough or sputum was noticed in the course of the disease. Since there is no improvement after anti-infection treatment, skin biopsy was performed for patients to make a definite diagnosis. While waiting for the examination results, the treatment plan was intravenous injection of methylprednisolone sodium succinate every day. The final diagnosis was “primary cutaneous extranodal NK/T cell lymphoma (nasal type)” by histopathology and immunohistochemistry. After that, the patient was transferred to oncology department for chemotherapy. Since its onset, it has been accompanied by fever and emaciation, without midfacial symptoms. The patient had a present history of brain infarction.
Physical examination showed that the patient’s vital signs were stable, no abnormalities were found in the nose and mouth, her left limb movement was limited, and his right mouth was skewed.
Dermatological examination showed that a walnut-sized red mass can be seen on the right shoulder (Figure 1). Part of the lesion had rupture scab. A circular-like infiltrated dark red plaque with a diameter of about 4cm can be seen on the right chest, with yellow and white dry secretion in the center (Figure 2). Multiple patches of dark red spots from coins to walnuts can be seen on the trunk and limbs (Figure 3).

Figure 1: Awalnut-sized red mass can be seen on the right shoulder part of the lesion had rupture scab.

Figure 2: A circular-like infiltrated dark red plaque with a diameter of about 4 cm can be seen on the rig
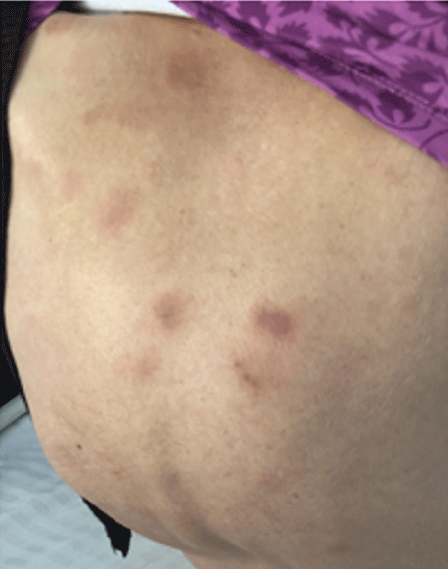
Figure 3: Multiple patches of dark red spots from coins to walnuts can be seen on the trunk and limbs.
Laboratory and auxiliary examination was as follows: blood routine: RBC 3.43 × 1012/L, HB 88g, WBC 0.9 × 109/L, PLT 89 × 109/L; coagulation series: plasma fibrinogen 1.26L. Lactate dehydrogenase 507U/L. Chest CT revealed inflammatory lesions in the lower lobes of both lungs. No obvious abnormalities was shown in rheumatism series, antinuclear antibody series and titer, superficial lymph node ultrasonography, abdominal ultrasound, brain, upper and lower abdomen and pelvic CT plain scan. Bone marrow routine indicated poor maturation of granulocytes, increased erythroids, and poor megakaryocyte production with iron deficiency.
Histopathological and immunohistochemical results of the skin lesion of the right shoulder: the tumor was comprised of lymphocyte with distinct atypia. Transparent cells, necrosis and mitosis can be seen in the tumor (Figures 4-6). Immunohistochemical staining revealed positivity for CD2, CD3, CD7, CD43, CD56 (Figure 7), TiA-1 (Figure 8), granase B (Figure 9) and vimentin. Immunohistochemistry revealed CD68 and lysozyme positivity in histiocytics. Some cells revealed positivity for CD20 (Figure 10), and a few cells revealed positivity for CD4, CD8. Immunohistochemistry demonstrated negative staining for CD30 (Figure 11). Ki67 index is 80% (Figure 12). Epstein barr virus in situ hybridization was positive (Figure 13).
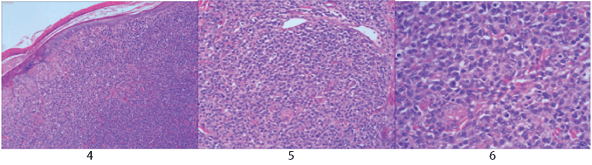
Figure 4-6: The tumor is composed of atypical lymphocytes to a certain extent, with transparent cells, necrosis and mitosis.
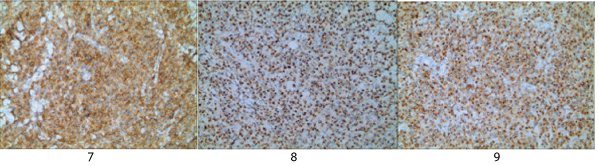
Figure 7-9: Immunohistochemistryy revealed positivity for CD56, TiA-1, granase B.
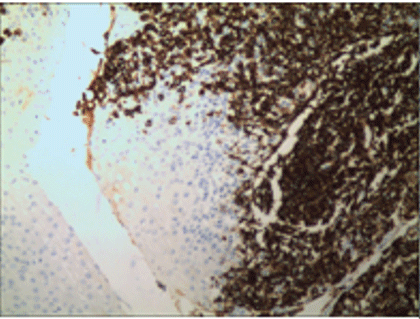
Figure 10: Some cells revealed positivity for CD20.
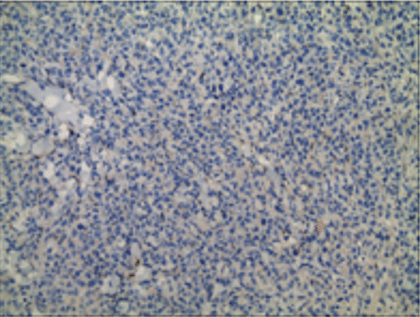
Figure 11: Immunohistochemistry demonstrated negative staining for CD30.
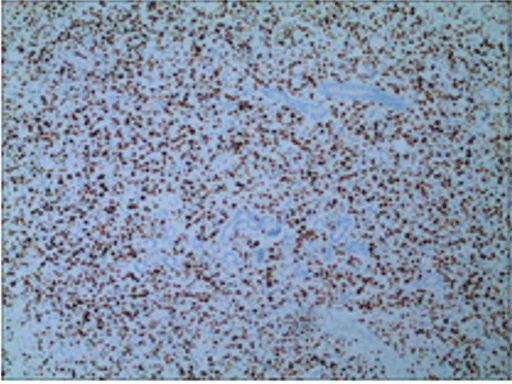
Figure 12: Positive expression of KI-67 in tumor nucleus (index 80%).
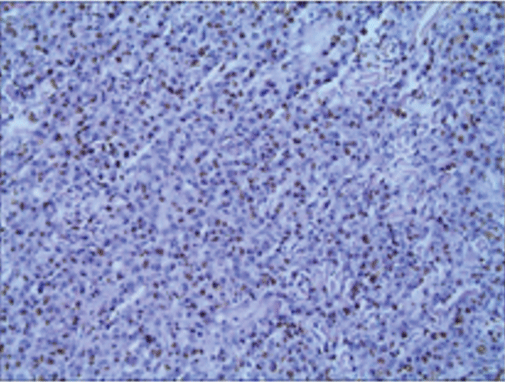
Figure 13: EBER positive tumor cells, in situ hybridization.
Combined with clinical manifestations, histopathology, immunohistochemistry and EBV in situ hybridization, the diagnosis was: primary cutaneous extranodal NK/T cell lymphoma (nasal type). After diagnosis, the patient was transferred to the department of oncology radiotherapy and chemotherapy for chemotherapy. After chemotherapy with perenterase, vincristine and methylprednisolone, part of the rash disappeared. Bone marrow suppression after chemotherapy developed 11 days later and the rash continued to improve. At 3 months of follow-up, the rash improved significantly (Figures 14 and 15), but she developed pneumonia and died of respiratory failure.
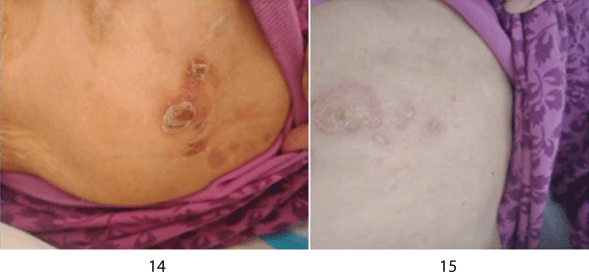
Figure 14-15: The rash on the right shoulder and chest was markedly improved.
Extranodal NK/T cell lymphoma, nasal type (ENKTL-NT) is a rare non-hodgkin’s lymphoma, which is highly aggressive. It accounts for about 2%~10% of all malignant lymphomas. Due to the majority of tumor cells expressing NK cell phenotype and the minority expressing cytotoxic T cell marker, this disease is called NK/T cell lymphoma. Enktl-nt is very unevenly distributed throughout the world and is more common in Asia and Central and South America. More than 80% of this disease occurred in nasopharynx and nasal cavity, and 26% occurred in skin, which is the most commonly involved extranasal site. When the skin is the primary site, it is called the primary cutaneous extranodal NK/T-cell lymphoma, nasal type (PC-ENK/TNT) [1]. In addition to the primary skin lesions, skin lesions may also be secondary manifestations of the disease. If the midline face is involved first, and then other anatomical lesions appear, it is a secondary or diffuse lesion [2]. Whether the skin lesions were primary or secondary, PC-ENK/T-NT is rare, which accounts for only about 0.3%~7.1% of cutaneous lymphoma [3].
The disease is associated with epstein-barr virus infection of T cells or NK cells, but the exact mechanism and its role in the pathogenesis of the disease have not been fully deciphered [4]. In recent years, other oncogenic mechanisms have been identified through high-throughput molecular and genomic mapping studies, including platelets-derived growth factor (PDGF), Janus-associated kinases/signal transducers and transcriptional activators (JAK/STAT), aurora kinase, NF-κB, and MYC, and have been identified as potential therapeutic targets [5]. The presence of tumor suppressor genes on chromosome 6Q21 may be associated with the pathogenesis, including HACE1, PRMD1, FOXO3 and PTPRK. In addition to inactivation/deletion of tumor suppressor genes, some oncogene activation may be associated with the pathogenesis of NK/T cell tumor, such as EZH2 and RUNX3 [6].
Extranodal nasal NK/T cell lymphoma has no characteristic skin lesion. And tumor tissue is often necrotic due to the histological characteristics of vascular destruction and coagulative necrosis, which makes clinical biopsy more difficult. Lesions are mostly single or multiple plaques, nodules, tumors and ulcers, often red or purple. Skin lesions are easily combined with local bacterial or fungal infection resulting in cellulitis, accompanied by fever easily misdiagnosed as infectious dermatosis. The disease can be accompanied by fever, discomfort, weight loss and other systemic symptoms. Hemophagocytic syndrome is also a possible complication of this disease [7].
The initial evaluation of a newly-diagnosed patient includes standard hematological and serum biochemistry profile, PET/CT and plasma EBV DNA quantification to rule out occult primary nasal lymphoma. Marrow trephine biopsy should be performed. With biopsy from bone marrow, we can confirm whether there is invasion to the bone marrow [8]. Random skin biopsy may be useful in early diagnosis, early tumor stage and assessment of prognosis in ENKTL-NT [9].
Diagnosis of ENKTL-NT depends on the pathological characteristics; immunohistochemical phenotypes and the evidence of Epstein-Barr virus infection. EB virus detection methods include EBER in situ hybridization, EBV immunophenotype, PCR detection of EB virus DNA and detection of EB virus antibody in peripheral blood. At present, the most common and sensitive method is in situ hybridization of EBV-encoded small molecule mRNA (EBER), whose detection rate is better than immunohistochemistry [10]. Studies have shown that peripheral blood EB virus detection has the significance of assisting in the diagnosis of NK /T cell lymphomas and evaluating the prognosis of patients [11], and EBV DNA load before and after treatment can predict short-term efficacy and long-term survival [12]. Histopathology tumor cells have characteristic vascular center growth pattern. Tumor cells infiltrate diffusely into the dermis and subcutaneous tissue, most of which are of moderate size. The tumor cells have irregular nuclei with mitotic patterns. Nucleoli are often inconspicuous, and faintly stained to translucent cytoplasm can be seen. Inflammatory cells such as plasma cells, eosinophils, small lymphocytes and histopathological cells are often found in histopathological manifestations, which mimic benign inflammatory skin disease and are misdiagnosed [13]. At present, no clinicopathological differences between different clinical manifestations have been found, but the possibility of differences cannot be ruled out [14]. Immunohistochemistry is the main diagnostic basis of ENKTL-NT. Tumor cells are bidirectional source of NK cells and T cells. Tumor cells express differentiation antigens of T-cell (CD3 and CD45RO) or differentiation antigen of NK cells (CD56, CD2 and CD36). It can also express cytotoxic proteins such as granzyme b, TIA-1 and perforin [15]. Clonal rearrangement of T cell receptor gene was found in a few cytotoxic T cell-derived patients. Ki67 is a marker to judge the degree of cell proliferation. The expression of Ki-67 index is inconsistent in NK/T cell lymphomas, and patients with high expression of Ki-67 usually have a poor prognosis [16,17].
Patients with cutaneous extranodal NK/T cell lymphoma usually have a survival of 2-15 months and a poor prognosis. A summary of previous reports indicated that patients with primary skin involvement had a better prognosis than those with secondary skin involvement [16], which may be related to the fact that the skin is prone to early skin lesions and can tolerate higher doses of radiotherapy [17]. Factors influencing the prognosis of ENKTL-NT generally include age, stage, lymph node involvement, extra-nasal involvement, B symptom, and elevated LDH level and presentation status. In a recent review, age, treatment strategy and treatment response were found to be independent and significant prognostic factors for cutaneous extranodal NK/T cell lymphoma [18].
Due to the rarity of cutaneous extranodal NK/T cell lymphoma and its clinical histological specificity, most cases are described in case reports or small series of reports, and there is currently no optimal treatment strategy. Extranodal nasal NK/T cell lymphoma is mainly treated with radiotherapy and chemotherapy in the early stage, with chemotherapy the main treatment for advanced ENKTL, and local radiotherapy can be considered for residual lesions. Allogeneic hematopoietic stem cell transplantation is often used as a salvage treatment, and the optimal time is the period when the disease reaches complete remission before transplantation, which is usually used for advanced and refractory/recurrent cases [19].
The patient is an elderly woman with early manifestations of a right shoulder mass, surface ulceration, scattered red plaques on the trunk and limbs, with fever, and with no manifestations on the face. She is in good mental state, with negative EB virus antibodies in peripheral blood, and her lungs at the early stage of onset partial infections can easily be misdiagnosed as infectious skin diseases. No pathogenic bacteria were found in bacterial cultures of secretions, with antiinfective treatments ineffective, and pancytopenia and progressive weight loss occurred. Skin tissue biopsy, immunohistochemistry, EBER in situ hybridization, and bone marrow aspiration were performed to finally support the diagnosis of primary skin extranodal NK/T cell lymphoma (nasal type). The disease is highly malignant. The patient’s age, B symptoms, increased LDH, and Ki-67 index of 80% all indicate a poor prognosis. Due to the age of the patient in this case, there are many underlying diseases. Because the right limb is unfavorable after cerebral infarction, and the skin lesions are scattered, therefore radiotherapy is not available. After chemotherapy, bone marrow suppression occurred after chemotherapy, and the rash continued to improve. However, she died of respiratory failure due to pneumonia during a 3-month followup. This case suggests that infections should not be the only focus on patients with skin necrosis and ulcers, lung infections, and repeated fevers after anti-infective treatment. Active search for the cause should be done and tumors and suspected rapidly progressing diseases should be ruled out. Skin tissue biopsy should be actively performed instead of waiting for the results of other non-invasive tests. Close attention should be paid because the disease progresses quickly. With the low incidence, it is easy to be misdiagnosed in the early stage.
Download Provisional PDF Here
Article Type: CASE REPORT
Citation: Lijie W, Feng J, Qian Z, Hui J, Jie B, et al. (2021) Primary Cutaneous Extranodal NK/T Cell Lymphoma (Nasal Type): A Case Report and Review of Literatures. J Clin Case Stu 6(6): dx.doi.org/10.16966/2471-4925.240
Copyright: © 2021 Lijie W, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access