Abstract
Primary Hepatic Neuroendocrine Tumors (PHNET) is a rare cancer less than 150 cases have been reported since its initial discovery by Edmondson in 1958. Many of these cases have been described in the literature using features of Computed Tomography (CT) or Magnetic Resonance Imaging (MRI) scans. However, 18F-fluorodeoxyglucose Positron Emission Tomography (18F-FDG-PET/CT) imaging been used less frequently. Only 10% of the hepatic neuroendocrine tumor cases characterized by hypermetabolic lesions were identified using PET imaging. Here, we report a case of PHNET with hypometabolic lesions observed by PET imaging with literature review. A 60-year old man presented with epigastric pain lasting for one month. An abdominal CT examination at a local hospital showed a hypodense lesion with intrahepatic bile duct dilatation in the hepatic caudate lobe, while the chest and pelvic CT were normal. Sonographic imaging showed a solid hypoechoic lesion in the caudate lobe of the liver along with an unclear boundary, a punctate blood flow signal, dilatation of the intrahepatic and extrahepatic bile duct, and right intrahepatic choledocholithiasis. PET/ CT imaging revealed hypometabolism which was due to a lesion in the caudate lobe and hence, primary hepatocellular carcinoma was initially diagnosed. Postoperative histopathology data indicated grade 2 PHNET. After surgery, the patient did not receive any further treatment in the subsequent 6-year follow-up period, during which he was in good health. PHNET is a low-grade malignant tumor for which surgical resection is the major treatment choice. PET/CT imaging plays a key role in evaluating tumor activity and preoperative differential diagnosis of primary and metastatic tumors.
Keywords
Fluorodeoxyglucose; Primary hepatic neuroendocrine tumor; Standardized uptake value
Introduction
A neuroendocrine tumor (carcinoid), also known as orargentaffinoma, is a malignant tumor derived from secretory neurons and amine-uptake precursor decarboxylated cells [1]. This type of tumor can occur in all areas of the body, but is most frequently found in the gastrointestinal tract and pancreas. A primary neuroendocrine tumor in the liver is extremely rare. Such a tumor was first described by Edmondson in 1958, and since then, less than 150 cases have been reported. Here, we report a case of Primary Hepatic Neuroendocrine Tumors (PHNET) identified using Positron Emission Tomography/Computed Tomography (PET/CT) imaging and review of the literature.
Case Report
A 60-year old man presented with epigastric pain, lasting for one month and with no obvious cause. Abdominal CT at a local hospital identified a location in the caudate lobe of the liver showing dilatation of the intrahepatic bile duct. The patient was then admitted to our hospital for a precise diagnosis and treatment. Sonographic imaging showed a solid hypoechoic lesion in the caudate lobe with an unclear boundary, a punctate blood flow signal, dilatation of the intrahepatic and extrahepatic bile duct, and right intrahepatic choledocholithiasis. No obvious abnormality was identified by either gastroscopy or colonoscopy. To determine the benign/malignant character of this lesion, a 18F-FDG-PET/CT examination was performed. After six hours of fasting and one hour after the injection of 251.6 MBq of 18F-FDG, the patient underwent a PET/CT examination using a dedicated scanner. The examination revealed a lesion measuring 5.8 cm × 6.2 cm in size with a CT value of 36.0 HU, indicating a mildly low density. The density of the parenchyma was homogenous, and the boundary was clear. PET imaging showed no 18F-FDG uptake by the hypodense lesion first detected by CT imaging in the caudate lobe. Based on these symptoms, this case was defined as a primary hepatocellular carcinoma. PET imaging identified no other primary lesions. Additionally, PET imaging confirmed the original sonographic findings of right intrahepatic choledocholithiasis, secondary to intrahepatic and extrahepatic bile duct dilation (Figure 1). Routine blood and liver function tests, and tests for tumor markers, such as carcinoembryonic antigen, α-fetoprotein, and Carbohydrate Antigen 19-9 (CA 19-9), were all within the normal range.
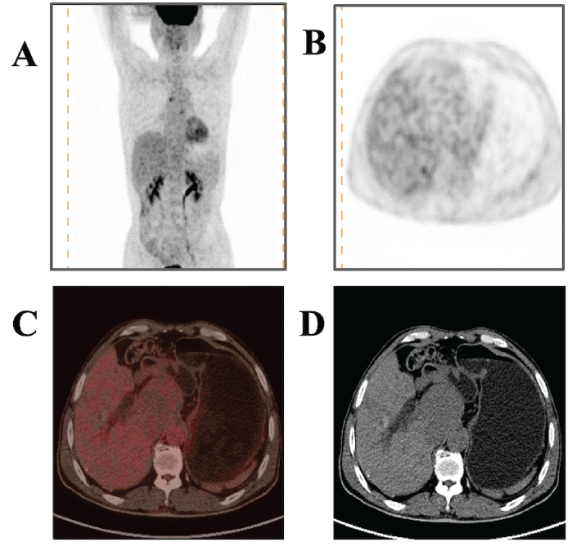
Figure 1: PET findings. (A) Maximum density projection image of PET. (B) The lesion showed 18F-FDG uptake in PET imaging with a maximum standardized uptake of 2.9, which was indistinguishable from that of normal liver tissue. (C-D) The mildly hypodense lesion measured 5.8 cm × 6.2 cm in size, had a clear boundary and was homogenous in density, and the adjacent inferior vena cava was compressed.
The patient underwent cholecystectomy, hepatic caudal lobectomy, anatomical extrahepatic lobectomy, choledochendysis, and T-tube drainage under general anesthesia. Neither ascites nor cirrhosis was observed; however, mild atrophy of the external margin of the left lobe was seen. The mass was located in the caudate lobe and left extrahepatic lobe, and no scattered nodules were seen in the abdominal pelvic cavities. No abnormalities were noted in the stomach, pancreas, or small intestine. Histopathology of the mass showed a well-differentiated grade 2 neuroendocrine tumor. The tumor cells showed a trabecular arrangement and were abundant in the interstitial blood sinus. Infiltration of the liver tissue and proliferation of surrounding fibrous tissue were observed, along with chronic cholecystitis. Immunohistochemical analyses results were positive for Chromogranin A (CgA), Cytokeratin 8 (CK8), and Synaptophysin (Syn), while they were negative for CK7, CK20, CD56, and GPC (Glypican); the Ki-67 immunopositivity value was 3%. These immune labeling results supported the diagnosis of PHNET (Figure 2).
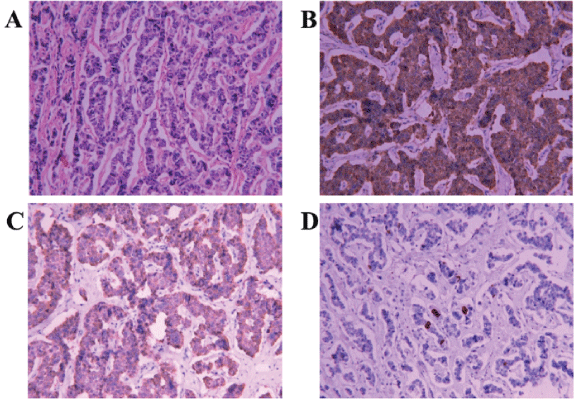
Figure 2: Histological manifestations of the liver neuroendocrine tumor. (A) The tumor cells showed a trabecular arrangement, the blood sinus of the mesenchyme was abundant, and liver tissue had been infiltrated by fibrous tissue hyperplasia (hematoxylin and eosin staining × 100). (B) Immunohistochemical staining of the tumor demonstrated immunoreactivity to CgA (× 200). (C) Immunohistochemical staining of the tumor demonstrated immunoreactivity to Syn (× 200). (D) Immunohistochemical staining of the tumor indicated a proliferation index of 3%.
The patient was discharged from hospital 28 days after surgery. At six-year follow-up, the CT examination was normal and NeuronSpecific Enolase (NSE) values were within the normal range, indicating that the patient was still in good health.
Discussion and Conclusion
Neuroendocrine tumors are malignant tumors derived from secretory neurons and amine-uptake precursor decarboxylated cells. Two possible origins of neuroendocrine tumors are the widely distributed neural crest and primordial endoderm. Neuroendocrine tumors can occur in all areas of the body, but are more common in the gastrointestinal tract and pancreas. Most liver neuroendocrine tumors are derived from gastrointestinal tumor metastases, with PHNET being extremely rare. Generally, neuroendocrine tumors are divided into three grade-based groups according to the malignancy potential of the tumor: grade 1, with less than two mitotic counts per ten high-power fields and/or a Ki-67 positive rate of <2%; grade 2, with mitotic counts between two and 20 per ten high-power fields and/or a Ki-67 positive rate between 3% and 20%; and grade 3, with more than 21 mitotic counts per ten high-power fields and/ or a Ki-67 positive rate of >20% [2]. Grade 1 tumors are low-grade well-differentiated neuroendocrine neoplasms characterized by hypometabolism and are, therefore, difficult to distinguish from the normal liver parenchyma metabolism by 18F-FDG-PET/CT imaging. However, they are also usually hypodense, a characteristic that can be detected by CT and Indium-111 octreotide scanning, the latter of which is a highly sensitive and specific technique for the diagnosis and staging of primary neuroendocrine tumors [3]. In intermediate-grade (grade 2) neuroendocrine neoplasms, the degree of 18F-FDG uptake varies greatly as they can show hyper- and hypometabolism. The degree of metabolism depends on the increase in Ki-67 expression. According to the diagnostic criteria, our patient had a grade 2 tumor that was, according to PET imaging, hypometabolic, possibly because of the low Ki-67 value. In comparison, many PHNETs in the literature that appeared as grade 2 tumors based on immunohistochemistry, showed hypermetabolism by 18F-FDG [4-6]. In poorly differentiated grade 3 neuroendocrine neoplasms, 18F-FDG-PET/CT and In111 octreotide scanning both contribute to an accurate diagnosis and staging [7]. Furthermore, compared with grade 1 and grade 2 tumors, grade 3 PHNETs have a higher proliferative activity, which influences patient prognosis [8]. The use of 18F-FDG is well-suited to glucose metabolism imaging but it is not an ideal tracer for detecting grade 1 and grade 2 neuroendocrine tumors in which it has a low diagnostic accuracy. In previous reports, only 57% and 66% grade 1 and grade 2 neuroendocrine tumors in patients from the literature, respectively, were positively detected by 18F-FDG-PET/CT. Although missed diagnoses and misdiagnosis can occur using 18F-FDG-PET/ CT imaging, it is still a more accurate method of detection than a conventional enhanced CT examination, which does not provide a sufficient diagnostic value for clinicians. In contrast, In-111 octreotide scanning and 68Ga-DOTA-somatostatin analogue-PET/CT are specific receptor imaging techniques for neuroendocrine tumors with a higher positive imaging rate for grade 1 and grade 2 tumors. They may also be useful for diagnosing and staging grade 3 tumors [9-11].
There are two main imaging manifestations of PHNETs: a single lesion and multiple concomitant secondary lesions. A single lesion tends to be large and well-circumscribed [12,13], with density heterogeneity, liquefaction, necrosis, and hemorrhage all being detected. For this tumor type, calcification is occasionally present, a pseudo-capsule is rarely seen, enhancement of the mass is not obvious, and a circular or significant diffuse heterogeneous enhancement is seen during the arterial stage. The degree of enhancement is lower than that of a primary hepatocellular carcinoma. In addition, both portal vein and hepatic vein tumor thrombi are rare for single lesions, and lymph node metastasis is rarer than when multiple lesions are present. In the case of multiple concomitant secondary lesions, they usually consist of a large lesion surrounded by multiple small lesions. The internal density of these lesions is heterogenous, and necrosis and hemorrhage can both be seen. They also show mild-to-moderate enhancement after contrast administration, making them difficult to distinguish from a metastatic tumor, and both portal fissure and retroperitoneal lymph node metastasis are common. In cases of multiple secondary lesions, it is difficult to distinguish PHNETs and secondary neuroendocrine tumors using conventional imaging. Although, Cha DI, et al. [14] demonstrated that PHNETs, characterized by their large tumor size, bleeding and hepatocellular carcinoma-like enhancement, could be distinguished from secondary neuroendocrine tumors using MRI. However, there are also limitations with this examination method and whole-body imaging is usually not performed, which may result in missing possible primary lesions. Although 18F-FDGPET/CT has low specificity in distinguishing PHNETs from other liver tumors, it can detect potential primary tumors, exclude extrahepatic metastatic lesions and provide reference values for subsequent clinical treatment.
PHNET is a low-grade and slow-growing malignant tumor. Surgical resection of PHNETs is the main treatment choice for those patients eligible for surgery [15,16] and early surgical resection results in a higher long-term progression-free survival and overall survival compared with other types of liver cancer [17-19]. Peptide Receptor Radionuclide Therapy (PRRT) using 90Y- and 177Lu-labelled peptides has been used over the last 15 years as a treatment option for neuroendocrine tumors, achieving approximately a 30% response rate [20]. An 18F-FDG-PET/ CT evaluation is useful for predicting the response to Lu-PRRT in patients with grade 1 and grade 2 neuroendocrine tumors [21]. In patients who are not eligible for surgery or those with recurrence, transcatheter arterial chemoembolization is the best treatment option with a 5-year survival rate ranging from 74-78% [22].
A final diagnosis of PHNETs depends on the postoperative pathological examination. Positive expressions of CgA, Syn, and NSE in immunohistochemical staining are important indicators for diagnosis of this disease. However, this present report indicates that PET/CT imaging plays a key role in the pre-operative differential diagnosis of primary and metastatic tumors, and a PHNET diagnosis should be considered in patients showing similar clinical symptoms to those of our case.
PHNETs are heterogeneous and the uptake of 18F-FDG varies greatly, with no uptake and only partial uptake usually observed in grade 1 and grade 2 tumors, respectively. Therefore, 18F-FDG-PET/CT is of limited value in the diagnosis of PHNET, especially for grade 1 and grade 2 tumors, but it is very valuable in evaluating tumor activity and distinguishing primary and metastatic lesions.
Funding
The present study was supported by the Chinese Academy of Sciences of “Biological basis and intervention strategy of brain aging” (Grant no. XDPB10).
Patient Consent for Publication
The consent for publication of the manuscript and the related patient images was obtained by the First Affiliated Hospital of University of Science and Technology of China.
Competing Interests
The authors declare that they have no competing interests.
Article Information
Article Type: CASE REPORT
Citation: Pan B, Wang SC, Chen ZK, Zou GC (2019) 18F-FDG-PET/CT Findings of a Primary Hepatic Neuroendocrine Tumor: A Case Report and Literature Review. J Clin Case Stu 5(1): dx.doi.org/10.16966/2471-4925.194
Copyright: © 2019 Pan B, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
Received date: 07 Sep, 2019
Accepted date: 25 Oct, 2019
Published date: 01 Nov, 2019



