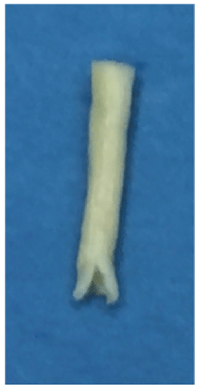
Figure 1A: Avance Nerve Graft. Undermining of the nerve graft epineurium allows for entabulation of the nerve end-to-end anastomosis


Basem T Jamal*
Assistant Professor and Consultant Oral & Maxillofacial Surgery/Head & Neck Surgical Oncology, King AbdulAziz University, Jeddah, Saudi Arabia*Corresponding author: Basem T Jamal, Assistant Professor and Consultant Oral & Maxillofacial Surgery/Head & Neck Surgical Oncology, King AbdulAziz University, Jeddah, Saudi Arabia, Tel: +966-555591789; E-mail: bjamal@kau.edu.sa
Trigeminal nerve injuries are associated with significant morbidity and reduced patient quality of life. Among the branches of the trigeminal nerve, such injuries most commonly affect the inferior alveolar nerve (IAN). However, the proximal and distal ends of the IAN cannot always be approximated to close gaps larger than 5 mm, necessitating nerve gap reconstruction. While autogenous nerve grafting is currently the gold standard for nerve gap reconstruction, such grafts have been associate with significant morbidity to the donor site. In the present report, we discuss the outcome of a patient who underwent nerve gap reconstruction following IAN injury using a decellularized human nerve graft.
Nerve graft; Allogenic; Inferior alveolar
Trigeminal nerve injuries are associated with significant morbidity and reduced patient quality of life. Patients report functional drooling while eating or drinking, which can negatively impact social functioning and self-esteem, as well as difficulty speaking and unintentional biting of the tongue and lip [1].
Injury to the peripheral branches of the trigeminal nerve has been well documented in the literature. Injuries to the inferior alveolar nerve (IAN) have been reported during dentoalveolar surgery, maxillofacial trauma and the subsequent surgeries for the repair of such trauma, orthognathic surgery, removal of benign and malignant tumors, dental implant placement, local anesthetic injections, and endodontic therapy [2-8]. Bagheri et al. [9] reported that the most common cause of IAN injury is third molar removal, followed by mandibular sagittal split ramus osteotomy and mandibular fracture.
While traditional nerve gap reconstruction utilizes autogenousnerve grafts, the use of such grafts has been associated with various comorbidities depending the site from which the graft is harvested [10,11]. In the present report, we discuss the outcome of a 27-year-old patient who underwent nerve gap reconstruction following IAN injury using a decellularized human nerve graft.
The patient was an otherwise healthy 27-year-oldwoman who had sustained injury to the right IAN during Lefort I and bilateral sagittal split osteotomies performed at an academic institution. The nerve endings were partially anastomosed due to contusion of the nerve ends, and orthognathic surgery was performed. Post-operatively, the patient reported complete numbness ofher lower lip and chin along the distribution of the IAN, which persisted for 6 weeks. Neurosensory examination revealed significant anesthesia, and oral examination at the one-month followup revealed erythema and pus discharge in the right posterior mandible region opposite to the plate fixation and the area of the nerve anastomosis. Based on these clinical findings, the patient was advised to undergo IAN microsurgery using allogenic nerve grafts.
The patient underwent IAN microsurgery 2 months after her initial injury. An intraoral vestibular incision was made extending from the anterior border of the ramus to the mental foramen. The titanium plate and screws were removed, following which the sagittal split osteotomy site was used to re-osteomatize the mandible and expose both the proximal and distal nerve ends. Further buccal window decortication was performed to expose the distal segment to allow excision of the neuroma and neurectomy of the nerve ends on both sides. A 1.5 cm gap was noted between the two nerve endings. An Avance nerve graft of 4-5 mm in diameter was passively adapted to the gap. Prior to implantation, the processed nerve allografts were completely thawed in either sterile saline or lactated Ringer’s solution for 5 to 10 minutes. The thawed allografts were implanted using the same microsurgical technique used for implanting an autograft nerve.
The graft epineurium was undermined 3 mm to allow for entabulation of the anastomosis site (Figure 1A). The epineuria of the proximal and distal ends of the IAN were sutured to the Avance graft at the inner end of the undermined epineurium using 7-0 Prolene sutures, followed by primary closure of the soft tissues (Figure 1B). Dexamethasone was administered for 2 days, following which the patient was discharged on a Medrol dose pack. She was also placed on a vitamin B and vitamin C regimen.

Figure 1A: Avance Nerve Graft. Undermining of the nerve graft epineurium allows for entabulation of the nerve end-to-end anastomosis
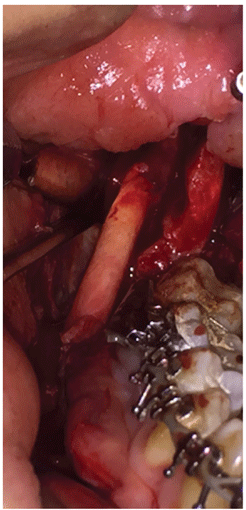
Figure 1B: Nerve Anastomosis. Avance nerve graft sutured to inferior alveolar nerve proximal and distal ends using 7-0 Prolene sutures
The postoperative course was uneventful. Surprisingly, at the 3-week follow-up, the patient reported regaining sensation along the distribution of the IAN. Neurosensory testing was performed using two-point discrimination, brush stroke identification, stimulus localization, and pin-prick sensation. Brush stroke identification and stimulus localization results were 3/10 and 2/5, respectively, and the patient had regained pinprick sensation along the lower lip vermilion and the medial half of the chin (Figure 2). The patient continued to exhibit gradual improvement over the following 3 months.
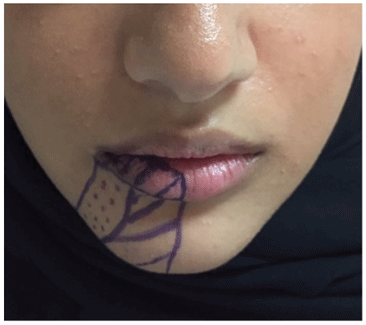
Figure 2: Map of the area in which the patienthad regained directional sensation and pin-prick sensation (lines), as well as the area in which she had regained directional sensation only (dots)
Unlike those of the lingual nerve, the proximal and distal ends of the IAN cannot always be approximated to close gaps larger than 5 mm. Longer nerve gaps repaired via direct neurorrhaphy will result in excessive tension over the suture line, which has proven to yield poor results in many studies [12-14].
Autogenous nerve grafting has been considered the gold standard for peripheral nerve reconstruction. However, extensive research regarding allogenic nerve grafts has yielded promising results, such that allogenic grafts may replace autogenous grafts as the gold standard for nerve gap reconstruction in the near future. Indeed, such a shift has been observed for sinus lift procedures, which previously relied primarily on autogenous grafts as well. Although allogenic nerve graft reconstruction was first proposed over two decades ago, nearly 18 months of post-operative systemic immunosuppression was required following early procedures [15-17].
Recent advancements have led to the development of decellularized human allograft products (e.g., Avance, AxoGen Inc, Alachua, FL, USA) [18], which are processed to retain the scaffold of nerve tissue but are non-immunogenic in the body. The use of decellularized nerve grafts has been associated with significant improvement in nerve repair outcomes, including associated with branches of the trigeminal nerve [19-22]. Furthermore, processed nerve allografts have been shown to be clinically effective and safe for peripheral nerve gaps ranging from 5 to 50 mm [23]. In one of the largest multicenter registries for peripheral nerve reconstruction, an overall 87.3% neurosensory recovery of peripheral nerves reconstructed with processed nerve allograft was observed based Medical Research Council Classification criteria for nerve injuries [24]. Zuniga also reported on the use of processed nerve grafts in the reconstruction of the trigeminal nerve following injury, observing a 788 % improvement in neurosensory scores for the IAN, with no adverse side effects. Moreover, Zuniga’s series also reported a 100% neurosensory improvement when repair was addressed within 90 days of the injury, although this rate decreased to 77% for repairs attempted after 90 days [21].
Several factors likely contributed to the positive outcome in our patient, including the time of repair. Although the influence of the timing of repair remainscontroversial, Ziccardi et al. [25] reported a significant association between nerve repair outcomes and the timing of repair, observing that early repair was associated with greater improvements in neurosensory function than late repair. Bagheri et al. [9] also reported that neurosensory function is inversely related to the time of repair, reporting significantly poorer outcomes for patients in whom repair is attempted 12 months after injury.
Another factor that likely contributed to the positive outcome is our patient was her young age. In several studies, Bagheri et al. demonstrated a negative association between advanced patient advanced and neurosensory recovery [7,8,26]. This finding is supported by the results of a recent study, which demonstrated that age-associated changes in electrophysiology and axonal transport negatively impact nerve repair outcomes [27]. Although the follow-up period was relatively short, our findings are significant due to marked recovery we observed in our patient in such a short time.
There are no conflicts of interest to declare.
Download Provisional PDF Here
Article Type: Case Report
Citation: Jamal BT (2017) Decellularized Human Nerve Graft in Allogenic Reconstruction of Inferior Alveolar Nerve Injury: A Case Report. J Clin Case Stu 2(2): doi http://dx.doi.org/10.16966/2471-4925.140
Copyright: © 2017 Jamal BT. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access