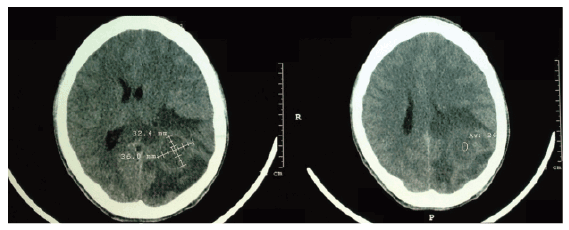
Figure 1: Skull CT without contrast showing vasogenic cerebral edema with mass effect, midline shift to the right and compression of the lateral ventricle and posterior horn of the left parietal region

Cláudia Maria Costa de Oliveira1* Eliseu Becco Neto2 Stelio da Conceição Araújo Filho2 Louize Emanuele de Oliveira Souza3 Larissa Costa O Santos4 Maria Luiza de Mattos Brito Oliveira5 Enrico Pinheiro de Oliveira6 Amanda Antunes Costa 7 Ronaldo Matos Esmeraldo8
1Physician, Renal Transplant Nephrologist at Hospital Geral de Fortaleza (HGF) and Professor at Uni Christus, Fortaleza, CE, Brazil*Corresponding author: Cláudia Maria Costa de Oliveira, Physician, Renal Transplant Nephrologist at Hospital Geral de Fortaleza (HGF) and Professor at Uni Christus, Fortaleza/ CE/Brazil, E-mail: claudiadrl@gmail.com
Cerebral tuberculoma is characterized by the formation of an expanding intracranial lesion that occurs as a result of hematogenous spread from a primary focus of Mycobacterium tuberculosis infection. Cerebral tuberculoma is a rare entity, constituting 5.5% of intracranial expansive processes observed in autopsies and there are few reports in the literature. The aim of this study is to present a case report of cerebral tuberculoma in a kidney transplant recipient and perform a literature review on the subject.
Central nervous system; Cerebral tuberculoma; Renal transplant; Headache
In the late nineteenth and early decades of the twentieth century, the cerebral tuberculoma had high incidence among intracranial expansive lesions, even in developed countries. This incidence of cerebral tuberculomas decreased from 30 to 3% in the mid-twentieth century due to the introduction of anti-tuberculosis chemotherapy [1].
Cerebral tuberculoma usually results from the hematogenous spread from an evident or latent primary focus of tuberculosis in other parts of the body [2]. The initial tuberculosis lesions may develop in the brain parenchyma or the meninges, and are known as Rich foci (caseous tubercles in the central nervous system where the bacilli could remain alive) [3].
Central nervous system (CNS) involvement occurs in 19.6% of patients with extra-pulmonary tuberculosis [4]. Intracranial tuberculomas are found in 0.5-5% of patients with tuberculosis and in developing countries, this percentage is higher, representing 15 to 20% of all brain tumors [5].
Recently, the prevalence of tuberculosis has been increasing due to the increased number of cases of acquired immunodeficiency syndrome, caused by the immunosuppression [6,7]. With the advent of transplants, especially kidney transplants, of which immunosuppression is mandatory and essential, the number of opportunistic infections has increased in recent years, culminating in the onset of rare infections such as cerebral tuberculoma. Due to the small number of reported cases of cerebral tuberculoma and the progressive increase in the number of cases, we decided to review aspects related to the epidemiology, diagnosis and treatment, based in the report of a clinical case.
R.A.M., male, 23 years old, was submitted to a kidney transplant from a living related donor 4 years ago. He received Sirolimus® and Sodium Mycophenolate® as maintenance immunosuppressive therapy. There was no acute rejection during post-transplant evolution and the patient used steroids only as induction therapy in the early transplant period (methylprednisolone 500 mg total dose)
He started to present high fever (up to 40°C) daily, with evening and nocturnal episodes without chills, which lasted for five days, in addition to right inguinal lymphadenopathy. He also reported back pain associated with the fever. Treatment with ciprofloxacin® was started due to suspected pyelonephritis, without improvement, followed by hospitalization to elucidate the case.
Exams at admission: Hemoglobin: 11.9 g/dl, Leukocytes: 12.700/mm3 (Eosinophils: 0.17%, Lymphocytes: 9.6%, Neutrophils: 82%, Monocytes: 7.3%), Platelets: 197.000/mm3 ; Creatinine: 1.9 mg/dl; Urea: 43 mg/dl; Na+: 136 mEq/l; K+: 3.1 mEq/l; AST: 15 UI/l; ALT: 11 UI/l. Urinalysis: proteins+, hemoglobin+, presence of leukocyte clusters. The patient was submitted to lymphonode biopsy, of which diagnosis was unspecific after hematoxylin-eosin, PAS, Grocot and Ziehl-Nielsen staining.
Other tests were performed to investigate the fever symptom: chest computed tomography (CT) disclosed the presence of infectious bronchiolitis, with “tree-in-bud” pattern into the anterior and lower segment of the left upper lobe and absence of mediastinal lymphadenomegaly. Blood cultures were negative. The tuberculin skin test was shown to be non-reactive. The serology for HIV, B and C Hepatitis was non-reactive. The transthoracic echocardiogram was normal.
Considering the strong possibility of pulmonary tuberculosis, the quadruple therapy for TB was initiated with rifampicin, isoniazid, pyrazinamide and ethambutol, followed by disappearance of fever and adenomegaly, and the patient was discharged. Two months later, the patient sought medical care again with clinical symptoms of severe headache, which had worsened in the last seven days, plus nausea, diplopia and right convergent strabismus. He showed no fever, weight loss or neurological deficits.
A skull CT was performed without contrast, which disclosed an extensive area of vasogenic edema, suggesting a mass effect with compression of the lateral ventricle and posterior horn of the left parietal region, diffuse effacement of cortical sulci and midline deviation to the right, as shown in figure 1.

Figure 1: Skull CT without contrast showing vasogenic cerebral edema with mass effect, midline shift to the right and compression of the lateral ventricle and posterior horn of the left parietal region
Magnetic Resonance Imaging (MRI) assessment disclosed a heterogeneous nodular lesion predominantly isointense on T1, hypointense on T2 and FLAIR, with restriction to diffusion, measuring 2.7 cm × 2.3 cm × 2.0 cm in the occipito-parietal transition, with apparent extension to the left corpus callosum, contralateral midline deviation with left ventricle collapse and unaltered posterior fossa, as shown in figure 2.
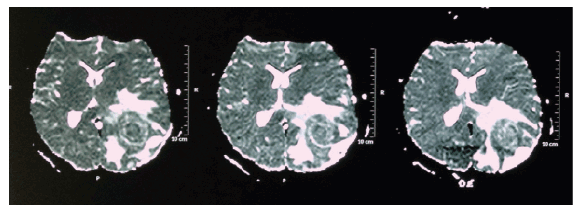
Figure 2: Magnetic Resonance Imaging (MRI) showing heterogeneous nodular lesion, predominantly isointense on T1, hypointense on T2 and FLAIR, with restriction to diffusion, measuring 2.7 cm × 2.3 cm × 2.0 cm in the left occipito-parietal transition, contralateral midline deviation with left ventricle collapse
The patient was submitted to microsurgery for brain tumor resection. He had a satisfactory evolution in the postoperative period, with progressive improvement of strabismus, visual acuity and headache. The postoperative skull CT confirmed complete resection of the lesion, as seen in figure 3.
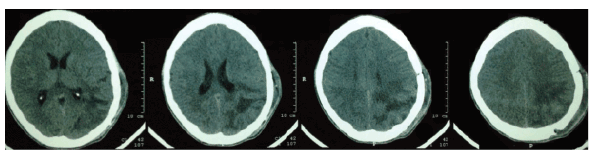
Figure 3: Skull CT in the postoperative period showing complete lesion resection
The histopathological analysis showed areas of necrosis with diffuse lymphocytic infiltrate suggesting cerebral tuberculoma (Figure 4). Special stains (Wade, Colt and Giemsa) were used, which suggested tuberculoma. The tuberculoma material was not cultured.
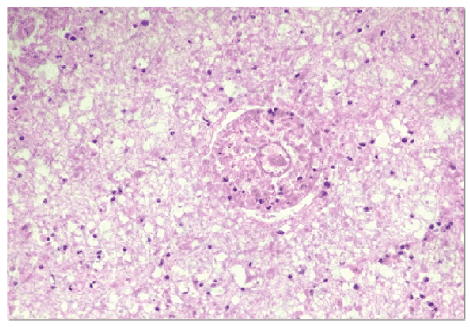
Figure 4: Histopathological analysis showing areas of necrosis with diffuse lymphocytic infiltration suggesting cerebral tuberculoma. (Hematoxylin-eosin staining)
Central nervous system tuberculosis can be classified into three clinical categories: tuberculous meningoencephalitis (tuberculous meningitis, cerebral tuberculoma and spinal arachnoiditis). Tuberculous meningitis is the most frequent manifestation of CNS TB, followed by CNS tuberculomas and tuberculous abscess [3].
Cerebral tuberculomas represent expansive intracranial lesions with extremely variable incidence, according to the literature reference. In most developed countries such as the United States and others in Western Europe, such incidences are significantly lower, amounting to 0.5 to 1% of expansive intracranial lesions [7]. In Brazil, isolated clinical cases of intracranial tuberculomas have been described. However, they constitute approximately 5.5% of intracranial expansive processes observed in autopsies [7].
The cerebral tuberculomas are usually solitary, with 15-34% being multiple [5,8,9] such as those described by Pittella and Toppa [10] in an alcoholic patient with 37 brain tuberculomas and can grow within the parenchyma or involve the meninges and parenchyma. The lesions of cerebral tuberculoma usually occur in the cerebral and cerebellar hemispheres because of their high vascular supply. In adults, they commonly occur in the frontal and parietal lobes [11]. Brain stem tuberculomas are rare.
The symptoms are non-specific and the size and location of the lesion determines the clinical findings, that may be related to mass effect [12]. Intracranial hypertension (papilledema/headache) may be present in 72% of patients [12-14], as well as focal neurologic deficits. Seizures occur in 50-85% of reported cases [14]. Signs of brain impairment expressed as meningeal irritation, increased intracranial pressure or signs of injury in cranial nerves (ptosis, strabismus) may occur. Constitutional symptoms such as fever, night sweats, and weight loss are usually absent [12,13].
The diagnosis of intracranial tuberculoma in the absence of extracranial tuberculosis can be quite difficult. The absence of tuberculosis characteristics on chest radiographs should not rule out the possible presence of a cerebral tuberculoma [15]. In a series of 70 patients with cerebral tuberculoma, only 30.8% showed a positive chest X-ray [16]. In 15-50% of cases, a lesion can be identified in the chest radiograph, compatible with active disease [7].
The tuberculin skin test, although usually positive, can be non-reactive as shown in our case, but a non-reactive test does not rule out the diagnosis, due to our patient’s use of immunosuppressive drugs [17]. Tuberculin skin testing is unreliable to aid in the diagnosis of tuberculoma. The result of a reactive test vary from 25% to 75% [17,18] and may be negative in these patients [19].
When tuberculoma is accompanied by meningitis, the diagnosis could be made early; otherwise, it may be missed [20]. Analysis of the cerebrospinal fluid (CSF) in cases of tuberculous meningitis shows hyper cellularity with lymphocytic predominance, but there may be neutrophil predominance in the acute phase of the disease. The CSF glucose is always decreased [5]. CSF smears for acid-fast bacilli (AFB) are positive in only 20% of the cases [21] and the sensitivity of PCR is 30%-80% [22]. CSF culture for Tb (gold standard test) takes a long time to complete and is negative in half of all patients. CSF analysis was not possible in the present case because of signs of severe intracranial hypertension at the diagnosis.
Biopsy of a suspected tuberculoma is necessary for definitive diagnosis frequently [23]. Both the culture and screening for alcohol-acid resistant bacillus in the tuberculoma material can be negative [17], highlighting the importance of the histological analysis, although pathology is not absolute [23]. Caseating granulomas with typical Langhans-type giant cells may be the only evidence of mycobacterial infection [13,23].
The skull CT was reported to have a sensitivity of 83-100% [2] and a specificity of 85.7%. However, when used alone, it does not allow diagnostic differentiation and cannot demonstrate the tuberculoma presence [24]. The lesion may present as round or lobed, a solid image with variable density, which, after contrast administration, can show a high-uptake halo of epithelioid cells, surrounded by a hypodense area of edema, as well as the presence of multiple lesions and meningitis [7]. The CT findings of brain tuberculoma during the acute phase of the disease, when performed without contrast, can disclose only a hypodense area caused by cerebritis or yield normal results.
At the established stage of granulomatous inflammation, the lesion can be isodense or more commonly hypodense, with a poorly defined outline in the pre-contrast images and enhanced after contrast use. In the central classification phase, the tuberculoma appears hypo dense, or less frequently, isodense and can be even slightly hyper dense in the pre-contrast images; rarely, small central calcifications can be identified. In the post-contrast images, an annular structure can be observed. These characteristics are not, however, pathognomonic of tuberculoma and can be seen in other circumstances, with the main differential diagnoses being cysticercosis, pyogenic abscess, gliomas, lymphomas and metastases [2,16,25]. In addition, the differential diagnosis depends on the tuberculoma development phase.
Magnetic resonance image (MRI) is reportedly superior to CT for diagnosis of brain tuberculomas and should be the technique of choice and/or histological diagnosis [2]. Without contrast enhancement on MRI the images are generally insensitive for detection of tuberculomas or meningeal inflammation [26]. In recent years, it has been reported that magnetic resonance spectroscopy of protons can be useful in differentiating tuberculomas from other intracranial lesions not elucidated by MRI [25].
In the present case, the patient underwent CT without contrast due to clinical urgency and the CT showed an image suggestive of unspecified left parietal brain tumor with mass effect, requiring CT with contrast and/or brain MRI to elucidate the case; the latter was then chosen, which confirmed a characteristic heterogeneous nodular image with mass effect and no meningeal lesions.
In case of clinical treatment, serial CT assessment shows the progressive regression of the lesion [27]. None of imaging modalities, including the MRI [28], can differentiate tuberculomas from other intracranial masses, although some authors have suggested that the presence of a central area of calcification (“target sign”) could be pathognomonic of tuberculoma [7], while others consider it to be non-specific and leading to misdiagnosis [11]. The case presented here showed no calcification area.
After the diagnosis has been established or is strongly indicative of cerebral tuberculosis, drug treatment should be chosen, which has a better prognosis than surgery [17]. Treatment schemes vary according to the patient’s age, the affected regions, bacterial resistance and the presence of HIV co-infection[29]. The literature suggests that patients with suspected CNS tuberculosis should undergo a prolonged course of anti-tuberculosis therapy, with a combination of at least four drugs (rifampicin, isoniazid, pyrazinamide and ethambutol) [30]. The optimal duration of treatment is unknown. There are reports with good results after 18 months of medical management [13]. The clinical response occurs even before the disappearance of radiological lesions [5].
It is noteworthy to report the possibility of paradoxical expansion of cerebral tuberculosis lesions during treatment with anti-tuberculosis chemotherapy [31]. As in the present case, other authors previously reported cases of patients with pulmonary tuberculosis who developed CNS involvement while improving the pulmonary disease with tuberculostatic treatment [32,33]. A possible explanation is linked to an immune phenomenon. The anti-tuberculosis drugs result in the destruction of mycobacteria structures and release of bacillus proteins and the latter would cause a hypersensitivity reaction. Secondary granulomatous vasculitis would occur in the perilesional area, associated with proliferation and vessel degeneration, with vessel lumen occlusion, which would hinder the anti-tuberculosis drug penetration into the lesions.
The use of corticosteroids for 4 to 8 weeks is part of the adjuvant therapy [23], with clinical improvement being observed in patients who develop intracranial hypertension or in whom the lesion has an important mass effect. The patient in this case report used dexamethasone for four weeks with significant clinical improvement. Evidence of new intracranial tuberculomas or the expansion of older existing lesions requires no change in the anti-tuberculous treatment. In such cases, systemic dexamethasone as adjuvant therapy for 4 to 8 weeks is effective [34].
Patients given empiric therapy with tuberculous medication may respond with a decrease in lesion size obviating the need of a biopsy in certain patient groups [35]. While clinical recovery takes place within a few weeks, radiological shrinkage or elimination of lesions lasts from 6-12 months [36].
If a lesion highly suspected of being a tuberculoma does not respond to appropriate chemotherapy, the diagnosis of pyogenic abscess can be considered and surgically treated. The pyogenic abscess is an unusual manifestation of CNS infection by Mycobacterium tuberculosis. The pyogenic abscess core consists of pus and abundant tuberculosis bacilli, differently from the tuberculoma, which depicts a solid caseous foci and few or no bacilli.
Stereotactic biopsy can be performed when there is no definite diagnosis, or when there is no response to therapy within 8 weeks, upon suspicion of tuberculoma [34]. The stereotaxic-guided surgery main advantages are the precise knowledge of brain structures, the exact location of the intracerebral lesions and less aggression to healthy brain tissue, thereby decreasing surgical morbidity. Without the stereotaxic approach in the case of small, subcortical and/or deep lesions, surgery may have unsatisfactory outcomes, considering the difficulty of intraoperative location of the lesion [7]. Nevertheless, the use of biopsy is controversial in the literature. Some authors believe that there may be dissemination of the infectious process. However, with the currently low morbidity of stereotactic biopsies (2%), it would be appropriate for such procedure to be performed in all cases, except those with active systemic tuberculosis. Mainly because Bouchama, et al. [12] observed an average of 2 cases a year of tuberculoma, while six patients that were initially diagnosed as tuberculomas had another diagnosis established after the biopsy (75% error rate). In some less typical cases, the biopsy may prevent lesion misdiagnosis (e.g meningioma) and prevent the patient from being exposed to the harmful effects of unnecessary therapy [37,38].
The surgical intervention may be necessary when there are acute complications such as hydrocephalus [34]. According to Artico et al. [18], the surgical option must be recommended in all symptomatic cases of single lesion. Surgical removal is indicated when the lesion location is accessible, as in the reported case, in which the patient showed clinical worsening even while receiving anti-tuberculosis drugs for nearly eight weeks and the lesion was potentially resectable, being submitted to resection through open surgery and total lesion removal [5].
The authors report the case of a young immunosuppressed patient, who developed probable pulmonary and lymphonode TB after 4 years of renal transplant and was submitted to empiric therapy, due to the high prevalence of tuberculosis in Brazil and due to chest tomography alterations suggestive of tuberculosis. Empiric therapy is practical in countries where the disease is highly prevalent.
There was no report of immunosuppressive therapy intensification due to acute rejection before the clinical picture and additional immunosuppression by HIV was not detected. Even after receiving the quadruple therapy for TB, the patient developed neurological symptoms compatible with cerebral tuberculoma, which showed good outcome after the abovementioned neurological conduct. It is possible that early CNS involvement was present at the time of the diagnosis of pulmonary tuberculosis, which went undetected by the clinical history and physical examination, due to the short time between the pulmonary lesion and the cerebral one. A paradoxical expansion of intracranial tuberculoma during treatment probably has ocurred in this patient.
Download Provisional PDF Here
Article Type: Case Report
Citation: Costa de Oliveira CM, Neto EB, da Conceição Araújo Filho S, Emanuele de Oliveira Souza L, Costa O Santos L, et al. (2016) Cerebral Tuberculoma in Renal Transplant Recipient treated in a Tertiary Care Hospital of Brazil - Case Report and Literature Review. J Clin Case Stu 1(2): doi http://dx.doi.org/10.16966/2471-4925.110
Copyright: © 2016 Costa de Oliveira CM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access