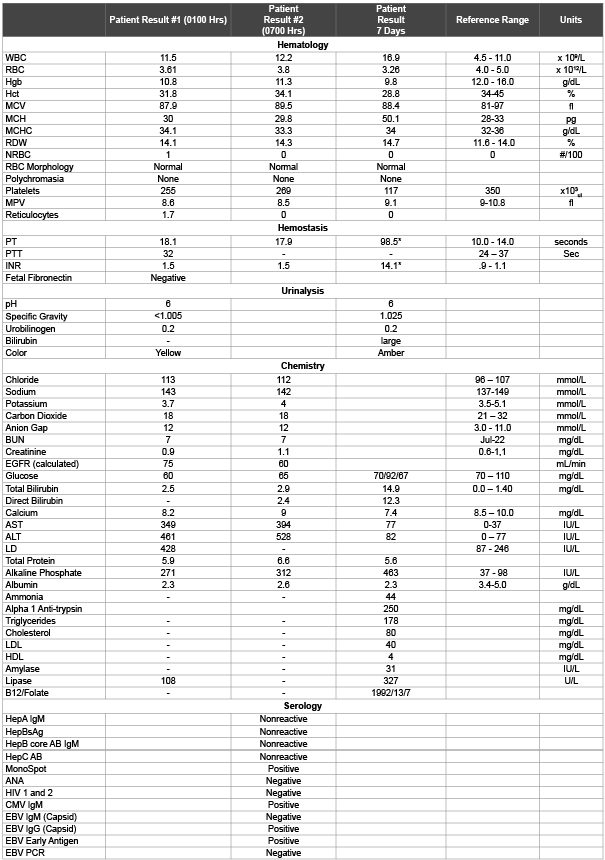
Table 1: Initial Laboratory Results
Note: * no anticoagulant therapy

Patricia M Tille1* Amanda K Graves1 Stacie Lansink1 Stephanie Jacobson2
1Medical Laboratory Science Program, South Dakota State University, USA*Corresponding author: Patricia Tille, Box 2202C, Brookings SD 57007, USA, Tel: 605-688- 6016; Fax: 605-688-6232; E-mail: pat.tille@sdstate.edu
HELLP Syndrome is a rare and serious complication of pregnancy. It is named for its characteristic presentation of microangiopathic hemolysis, elevated liver enzymes and low platelet count. Disease symptoms include right upper quadrant pain, epigastric pain, nausea, vomiting, malaise and headache in addition to preeclampsia. HELLP Syndrome is associated with abnormalities in the development of the placental vasculature resulting in poor perfusion. This creates a hypoxic state, resulting in anti-angiogenic proteins being released from the placenta, ultimately hindering proper fetal development. The subsequent placental damage results in maternal endothelial cell dysfunction, leading to platelet activation and fibrin deposition in the liver causing tissue necrosis. If left untreated, HELLP Syndrome can lead to liver failure and death of the mother and fetus. The following case study presents an atypical case of HELLP Syndrome in a Native American female following a fall at 32 weeks of gestation.
HELLP Syndrome; Microangiopathic hemolysis; Preeclampsia; Antiangiogenic proteins; Necrosis
CBC: Complete Blood Count; CMP: Complete Metabolic Panel; PT: Prothrombin Time; INR: International Normalized Ratio; aPTT: Activated Partial Thromboplastin Time; LD: Lactate Dehydrogenase; AST: Aspartate Aminotransferase; ALT: Alanine Aminotransferase; DIC: Disseminated Intravascular Coagulation
A 27 year-old Native American female presented to the Emergency Department at 32 weeks gestation complaining of abdominal pain. The patient reported vomiting and a gradual increase in pain during eating or drinking. The onset of symptoms developed as a result of a fall down a flight of stairs two weeks prior, however the patient failed to seek medical care. The patient’s medical history included four previous pregnancies, two of which were live births, an abnormal pap smear that was Human Papillomavirus (HPV) positive, and a surgical lung repair due to a stabbing incident. The patient denied illicit drug or alcohol use and reported daily use of tobacco. The patient’s vitals and preliminary exam are unremarkable except for the following: a soft tender lower abdomen upon palpation, extremities show signs of mild, trace, pitting edema, lung examination showed shallow breathing and eyes appear yellow (Icteric).
Initial diagnostic testing included an ultrasound, complete blood count (CBC), prothrobmin time (PT), activated partial thromboplastin time (aPTT), complete metabolic panel (CMP), lipase, urinalysis and culture. Abnormal laboratory results are presented in table 1. The laboratory data indicates that the patient has a significant increase in liver enzymes, a slight decrease in hemoglobin, a decreased red blood cell count and an increased protime. The patient’s initial laboratory results and symptoms indicate a hemolytic condition, a decrease in coagulation efficiency, liver dysfunction and generalized inflammation suggestive of preeclampsia and HELLP syndrome.
After several hours in the emergency department, the patient was transferred to obstetrics for further evaluation. The obstetrician ordered a follow-up CBC with differential, PT, and CMP. In addition, the physician ordered a toxicity test for Acetaminophen, ANA (Anti-nuclear Antibody) Screen, a fetal fibronectin and a hepatitis panel (Hepatitis A, B and C).The toxicity screen for acetominophen (<2.0 ug/mL, reference range 10-30 ug/mL) in addition to the repeat CMP provides an indication of whether the liver function is improving or declining as a result of a drug induced condition. The hepatitis panel was also to determine if the liver dysfunction could be associated with a viral infection. Fetal fibronectin levels, a protein that maintains attachment of the amniotic sac, are used to predict potential preterm delivery of the fetus. The breakdown and subsequent appearance of fetal fibronectin in the mother’s vaginal discharge would be an indication that the patient is at increased risk for preterm labor. The patient’s fall down the stairs presents a concern to the physician that the baby could be at risk due to damage to the placenta or dislodging of the amniotic sac. A fetal non-stress test and ultrasound were also performed to ensure that the fetus was not under any undue stress.
The patient’s suspected diagnoses including preeclampsia and HELLP syndrome are associated with a systemic inflammatory response. The ANA Screen was used to determine if the patient’s symptoms were associated with an exacerbated case of Lupus Erythematosus or another inflammatory condition. The patient had a slightly elevated white blood cell count; however the differential was within normal range. This indicates that there appears to be no infection and the slight elevation is likely due to a generalized inflammatory response. The red blood cell count is decreased, along with the hemoglobin and an increase in the red cell distribution width and presence of nucleated red blood cells indicates that the patient is producing new red blood cells, however at a rate that is insufficient to counteract the associated pathology (potential uncontrolled bleeding) or a hemolytic process that is occurring. The protime and activated partial thromboplastin time and INR are ordered in conjunction with the CBC to determine if the patient is experiencing any unexplained bleeding that is causing the decrease in the red blood cell count. The elevated PT and INR indicate that the patient’s blood is not clotting fast enough. As the initial laboratory results indicated no decrease in platelets, these results would support a diagnosis associated with liver dysfunction. The elevated LD, AST, ALT and alkaline phosphatase support a potential case of injury associated liver damage or acute liver disease. In addition, the patient’s reported symptoms including the yellow discoloration of the eyes, along with the nausea and vomiting are common symptoms of acute liver disease. The patient also appears to present with rapid shallow breathing as demonstrated by the elevated chloride and low carbon dioxide levels. Abnormal laboratory results are presented in table 2.

Table 1: Initial Laboratory Results
Note: * no anticoagulant therapy
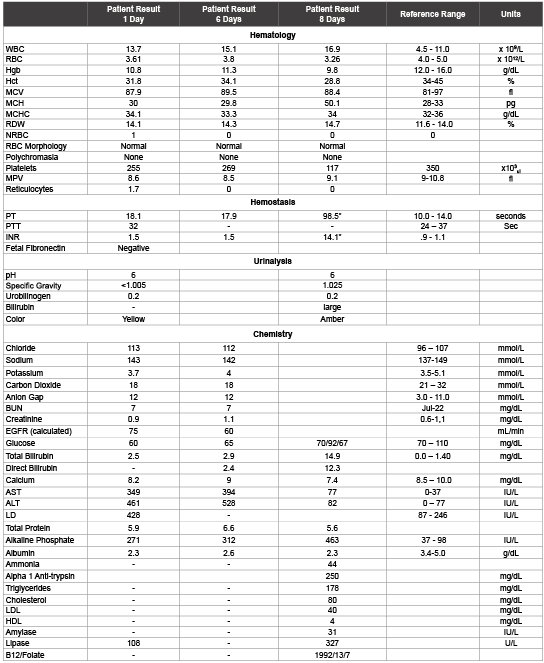
Table 2: Post Delivery Laboratory Results
The attending obstetrician was able to rule out any bleeding or internal damage in the patient as a result of the fall down the stairs, liver disease from viral or other infectious processes and systemic autoimmune disorders. Based on the patient’s condition and length of pregnancy, the symptoms and laboratory results indicate that the patient was suffering from late on-set preeclampsia leading to HELLP Syndrome. The patient was kept overnight for observation and sent home the following day with orders of strict bed rest with the hopes that preterm delivery of the fetus could be avoided and that the mother’s condition would resolve independently. Over the course of the next three days the patient was seen daily in the emergency department for abdominal pain. The patient was re-admitted to the hospital on the third day for delivery of the fetus and emergency transfer to a tertiary liver center.
Several disorders demonstrate similar laboratory test results and symptoms as HELLP syndrome making differential diagnosis difficult. It is important for the physician to rule out other conditions such as hemolytic anemia, thrombotic microangiopathies, acute fatty liver of pregnancy and system lupus erythematosus [1].This is essential in order to determine proper treatment and prevent serious injury to both the mother and the fetus as a result of an undiagnosed case of preeclampsia and HELLP syndrome. Patients who present with microangiopathic disorders and hemolytic anemia may be successfully treated through the use of transfusion therapy with plasma, red blood cells or platelets. Patients with acute fatty liver pregnancy and severe preeclampsia however often require preterm fetal delivery. It is however possible to delay delivery of the fetus in successfully treated microangiopathic disorders, hemolytic anemia and the exacerbation of lupus [1].
HELLP Syndrome results as a complication from severe preeclampsia (hypertension, fluid retention, excessive weight gain and increased urine protein) and typically includes hemolysis of red blood cells; elevated liver enzymes and a low platelet count [2-4]. Preeclampsia usually manifests with symptoms including fatigue, excess weight gain, headache, nausea with vomiting, pain in the upper right abdominal quadrant, blurred vision, nosebleeds or other uncontrolledbleeding, enlarged liver, high blood pressure, icterus (yellowing) of the eyes and seizures [5]. Patient’s that present with the initial symptoms of preeclampsia have a 4-12% chance of developing HELLP Syndrome [2]. The disease pathology in the development of HELLP Syndrome is a result of placental vascular insufficiency caused by maternal endothelial cell dysfunction that leads to platelet activation and fibrin deposits during the 27th and 37th week of gestation [5-7]. The red blood cell hemolysis occurs as the cells are fragmented as they pass through damaged endothelial vessels [10]. The cellular damage results in the microscopic appearance of spherocytes, schistocytes and burr cells in the peripheral blood smear [8]. Liver enzymes, specifically Lactate Dehydrogenase (LD), aspartate aminotransferase (AST), and alanine aminotransferase (ALT) are increased as a secondary condition due to fibrin deposits in the vessels causing obstruction of the hepatic blood flow and therefore liver dysfunction [2]. The lack of blood flow to the liver results in tissue necrosis of the liver [8]. The tissue damage prevents the liver from properly producing a variety of factors required for coagulation resulting in a decrease in necessary clotting factors. This is accompanied by a decrease in platelet count (thrombocytopenia) as a result of the increased consumption as well as platelet activation caused by the endothelial cell damage [2]. In addition to the pathological responses that decrease platelet and clotting factors, pregnancy also creates a physiological strain exerted on the endothelium resulting in an increase in platelet aggregation and a decrease in the number of circulating platelets. Uteroplacental consumption of platelets has also been implicated in the reduction of circulating maternal platelets [9]. A complication in the diagnosis of HELLP Syndrome occurs because the patient may present with one or all of the associated symptoms [2].
HELLP syndrome, as previously indicated typically follows pregnancy-associated preeclampsia. Preeclampsia is also a multisystem disorder and several molecular factors have been identified that are associated with the development of preeclampsia and HELLP syndrome. Mutations and altered function in a variety of molecules have been linked to both conditions in a variety of studies including the Factor V Leiden mutation, metabolism of methionine homocysteine, Factor II (Prothrombin) mutations, polymorphism of the glucocorticoid receptor gene, microsomal epoxide hydrolase gene, vascular endothelial growth factor, polymorphisms in the angiotensin-converting enzyme and other transporters or receptor genes [6]. Various pro-angiogenic factors are produced in order to create sufficient blood profusion to the fetus in utero. However, the chronic inflammation that occurs in preeclampsia activates anti-angiogenic factors that then counteract the physiological effects of the angiogenic factors creating vasodilatation and endothelial dysfunction leading to hypertension. The discussion of the molecular mechanisms associated with the development of preeclampsia and HELLP syndrome is highly complex and beyond the scope of this case presentation.
The underlying cause for preeclampsia leading to HELLP syndrome begins with reduced placental perfusion as a result of the inhibition of angiogenic factors in the maternal bloodstream. The decrease in placental perfusion will then exacerbate existing maternal conditions including hypertension, vascular resistance, renal disorder, diabetes and metabolic syndrome. The systemic effect results in the release of metabolic activators that induce generalized inflammation. One of the major immune modulators of generalized endothelial activation is IL-6. Studies indicate that IL-6 levels are differentially altered in early versus late onset preeclampsia [12]. This is one of the factors that allow preeclampsia and/ or HELLP syndrome to be classified as early onset (first trimester) or late onset (third trimester) [6]. In addition, current data indicates that women with early onset preeclampsia demonstrate a higher risk for fetal mortality than women with late onset preeclampsia and HELLP syndrome [7].
The ability to quantitate immune modulators such as IL-6 to identify preeclampsia and provide early treatment is relatively new. Serology along with the development of new technologies and molecular diagnostics that includes a multitude of techniques in genomics, transcriptomics, proteomics, and metabolomics are now being utilized to improve the diagnosis to the pathogenesis and prognosis for patient outcomes in cases of preeclampsia and HELLP syndrome. Microarray analysis of 43 genes has demonstrated a unique differential expression of 31 genes from whole blood specimens in early-onset preeclampsia and uncomplicated pregnancy. The majority of these genes are involved in coagulation, immune regulation and development, whereas seven genes are implicated in the innate nonspecific immune response and cell-to-cell recognition of the nervous system. Among the major differences are genes essential to host defense, tight junctions in the blood-brain barrier and liver regeneration [10]. These differences clearly point to the metabolic changes associated with nervous system alterations, and metabolic dysfunction that occur in severe cases of HELLP syndrome associated with severe preeclampsia.
HELLP syndrome may also be divided into classes. A representative classification scheme is presented here and is based on categories established by the University of Mississippi. Class I: severe thrombocytopenia (platelets <50,000/uL) with evidence of liver dysfunction with an AST or ALT ≥ 70 IU/L and evidence of hemolysis (total serum LDH >600 IU/L) with a maternal morbidity rate of 64%. Class II: moderate thrombocytopenia (platelets 50,000 to 100,00/ uL) with other results similar to Class I with a maternal morbidity rate of 54%. Class III includes mild thrombocytopenia, mild liver dysfunction with an AST and/or ALT > 40 IU/L and hemolysis (total serum LDH>600 IU/L) [11,12]. There is significant variation in outcomes for patients that develop HELLP Syndrome from adverse maternal to adverse fetal complications (Table 3).
The severities of the symptoms and potential maternal and fetal complications that may arise from the disease are of significant concern. Delivery of the fetus is recommended in diagnostic cases of HELLP syndrome prior to 34 weeks gestation if the mother’s condition does not improve rapidly following treatment. Delivery is typically scheduled within 24-72 hours following diagnosis following treatment with corticosteroids, magnesium sulfate and systolic blood pressure control [5,8,12]. Steroids are organic compounds that contain cycloalkane rings designed to assist in the improvement of a number of different physiological functions [13]. Corticosteroid improve fetal lung maturation resulting in a decrease in the occurrence of fetal respiratory distress syndrome and accelerates maternal recovery from HELLP syndrome following fetal delivery. This ultimately results in a decrease in maternal morbidity [13]. In addition to steroid treatment; patients currently receive intravenous magnesium sulfate. The compound is said to “neuroprotect” the patient by binding proteins, activating enzymes, improving membrane integrity and calcium balance, and is involved in cell signaling, cell division and apoptosis. Patients who fail to recover following delivery and treatment with corticosteroids and magnesium sulfate are treated with plasma exchange with fresh frozen plasma to replace the fluid and reverse the pathophysiology associated with the changes in the peripheral blood components [12].
The initial condition that was affecting this patient upon admission was preeclampsia based on a physical exam that indicated an elevated blood pressure, proteinuria and no previous identifiable risk factors. Preeclampsia usually manifests with symptoms including fatigue, excess weight gain, headache, nausea with vomiting, pain in the upper right abdominal quadrant, blurred vision, nosebleeds or other uncontrolled bleeding, enlarged liver, high blood pressure, icterus (yellowing) of the eyes and seizures. The condition is most often associated with a woman’s first pregnancy [5]. More specific signs of preeclampsia include a high ALT, AST and LD level, microangiopathic hemolysis and a decrease in platelet count [2]. The patient described in this case presented to the emergency department with many of these symptoms. The patient had several previous pregnancies with no history of hypertension or pregnancy associated complications or risk factors or conditions typically associated with the development of preeclampsia or HELLP syndrome. Development of one or both of these conditions are associated with risk factors that typically include chronic hypertension, renal disease, diabetes mellitus, obesity and a family history of preeclampsia [6,7]. The patient did not indicate that she had experienced any symptoms or show signs of maternal or fetal complication prior to her fall down the stairs. The patients’ CBC showed a decreased red cell count that is consistent with HELLP syndrome. The red cells are being fragmented as they pass through the fibrin coated veins. Additionally the patient had no prior history of liver damage but presented with elevated AST, ALT and LD levels that are consistent with HELLP Syndrome. In a typical diagnostic scenario, the patients’ platelet count would also be decreased. However the patients’ initial laboratory results did not show a decrease in platelet count. The patients’ platelet count was only slightly decreased upon her final visit to the emergency department three days later. However, 7 days post admission to the emergency room, the patient’s platelet count took a dramatic turn, decreasing significantly. This indicates that the patient had transitioned from preeclampsia to HELLP Syndrome during the week following her fall that prompted her initial emergency room visit.
At the time of the initial examination, the patient was well into the third trimester of pregnancy. The patient was in between what would be considered early onset preeclampsia (occurring prior to 33 weeks) and late onset preeclampsia that occurs 33 to 34 weeks. Late-onset preeclampsia is atypical and potentially life threatening [4,14]. Severe late preeclampsia is associated with high mortality and catastrophic nervous system events [8]. The diagnostic criteria for both early and late-onset preeclampsia are the same, however they are characterized by different clinical features but more importantly are associated with differences in the severity of maternal and fetal outcomes [4,14].
When the patient returned to the emergency department on the third and final visit 7 days post trauma, she was in labor and delivery of the fetus was imminent. During the delivery of the fetus, the patient experienced significant blood loss that resulted in transfusion of blood products before being transferred to the tertiary liver disease center for proper treatment of HELLP Syndrome. Immediately following the delivery of the fetus the patients’ liver enzymes began to improve rapidly and neither the mother nor the fetus experienced long-term effects from the disease.
The fact that the patient and the newborn experienced no long-term effects is significant in this case. It would appear that the injury might have triggered an increased inflammatory response that exacerbated the existing preeclampsia leading to the development of HELLP Syndrome. In addition, there is no disease data available that indicates the frequency or severity of HELLP syndrome in Native Americans. Current literature indicates that HELLP Syndrome is reported to be more predominant in the Caucasian population than that of other ethnicities. Speculation as to the reason for the predominance in the Caucasian population may be a lack of reported cases [5,12]. The lack of reported cases and difficulty diagnosing preeclampsia in non-symptomatic patients makes it extremely difficult to correlate the multifactorial disease pathophysiology to a single root cause. Continued awareness, education and analysis of patient data are needed in order to provide continued successful early diagnosis and continue to improve maternal and fetal morbidity. Subsequently the next challenge is to identify ways to effectively prevent preeclampsia and HELLP syndrome.
Download Provisional PDF Here
Article Type: Case Report
Citation: Tille PM, Graves AK, Lansink S, Jacobson S (2015) A Case of Injury Associated HELLP Syndrome in a Native American. J Clin Case Stu 1(1): doi http://dx.doi.org/10.16966/2471-4925.101
Copyright: © 2015 Tille PM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access