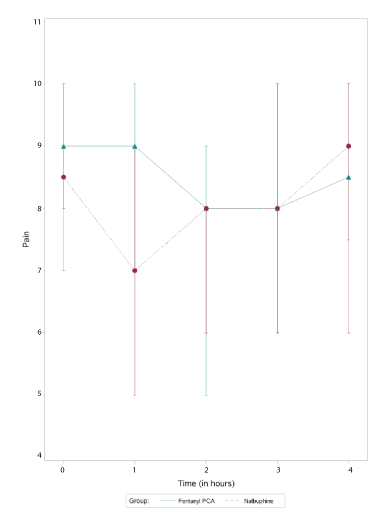
Figure 1: Median pain scores vs time.

Truc-Anh T Nguyen1* Xiao-Feng Wang2 Karl Wagner3 Marcos Izquierdo3 Norman Bolden3
1Department of Anesthesiology and Critical Care Medicine, Johns Hopkins Hospital, Baltimore, Maryland, USA*Corresponding author: Truc-Anh T Nguyen, Assistant Professor, Department of Anesthesiology and Critical Care Medicine, Johns Hopkins Hospital, 1800 Orleans Street, Sheikh Zayed Tower 8120, Baltimore, Maryland 21287, USA, Tel: 410-955-5608; E-mail: tnguy1932@jhmi.edu
Article Type: RESEARCH ARTICLE
Citation: Nguyen T-AT, Wang XF, Wagner K, Izquierdo M, Bolden N (2018) Labor Analgesia when Neuraxial Anesthesia is Relatively Contraindicated: Comparison of Patient-Controlled Fentanyl and Intermittent Nalbuphine Boluses. J Clin Anesth Manag 3(1): dx.doi. org/10.16966/2470-9956.138
Copyright: © 2018 Nguyen T-AT, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
Background: A patient-controlled fentanyl protocol for parturient with relative contraindications to neuraxial anesthesia was implemented. The primary goal of this study is to identify any increased risk for adverse events to the mother or fetus associated with our fentanyl PCA protocol. The primary outcome studied was maternal/fetal adverse events. Secondary outcomes studied included verbal pain score (VPS) during labor and incidence for adherence to the specified protocol.
Methods: A single-center chart review of patients utilizing patient-controlled fentanyl for labor from August 2009 through August 2015 was performed to determine maternal/fetal adverse events and pain control. This group was compared to a similar group receiving intermittent nalbuphine boluses for labor analgesia.
Results: There were no maternal complications observed in either group, and fetal adverse events were not significantly different between the fentanyl vs nalbuphine groups. There was a significant decrease in the verbal pain score at 2 hours in the fentanyl group (p=0.0180) and at 1 and 2 hours in the nalbuphine group (p=0.0001 and p=0.0157, respectively). The pain score was lower in the fentanyl group at 2 hours compared to the nalbuphine group. The verbal pain scores were unchanged at 3 and 4 hours in both groups compared to baseline.
Conclusion: Maternal and fetal adverse events related to narcotic therapy during labor were very uncommon. Patient-controlled fentanyl is a safe and reasonable option for labor analgesia in settings where epidural analgesia is relatively contraindicated, or not desired by the parturient.
Labor fentanyl PCA; Neuraxial anesthesia Contraindications; Labor analgesia
Neuraxial analgesia is the gold standard for providing maternal pain relief during labor [1]. While the majority of patients in the United States opt for neuraxial analgesia for labor pain relief [2], there are a number of distinct circumstances that are considered an absolute or relative contraindication to the performance of neuraxial anesthesia [3]. We implemented a fentanyl patient-controlled analgesia (PCA) protocol at our institution in 2009 to help guide obstetricians and standardize the care for patients who were unable to receive epidural analgesia due to relative contraindications to neuraxial anesthesia. Nurse administered intermittent intravenous (IV) 5mg nalbuphine boluses (by order of obstetricians) are routinely used at our institution for patient’s not receiving epidural analgesia. The number of patients at our institution that elect not to receive either neuraxial analgesia or IV opioid therapy for labor pain relief is almost nil.
The primary goal of this study is to identify any increased risk for adverse events to the laboring patient or her fetus associated with the implementation of our fentanyl PCA protocol. The primary outcome studied was maternal/fetal adverse events associated with fentanyl PCA use during labor. Secondary outcomes studied included verbal pain score (VPS) during labor and incidence for adherence to the specified protocol.
Approval was obtained from the Institutional Review Board of Metro Health Medical Center at Case Western Reserve University for this retrospective cohort study. The study was exempt from requiring informed consent. We implemented a labor fentanyl PCA protocol in August 2009. Conditions specified in our protocol that might justify use of the fentanyl PCA protocol included coagulopathy, current anticoagulation therapy with therapeutic effect, patients with anatomic defects in the spine, and patients with severe symptomatic spine pathology. The labor fentanyl PCA was only to be offered to parturient that had a relative contraindication to neuraxial anesthesia. Our protocol consisted of an initial bolus of 50-100 mcg of IV fentanyl with initial PCA settings allowing 50 mcg bolus doses every 10 minutes with an initial 1 hour maximum dose 250 mcg. The amount of fentanyl delivered could be increased by the physician based on patient response. Our protocol specified that a neonatal resuscitation team was to be summoned and be in attendance for all patients that delivered while receiving a fentanyl PCA for labor, and naloxone was to be immediately available for use by the code pink team.
Pharmacy records were reviewed to identify all patients that utilized intravenous fentanyl PCAs in the Labor and Delivery Suite after implementation of our fentanyl PCA protocol for labor (August 2009-August 2015). We identified 52 patients that used the fentanyl PCA during our study period. The medical records of these patients were abstracted to collect data on patient characteristics, indication for fentanyl PCA, verbal pain scores (VPS) during labor on a scale 0-10, and maternal/fetal adverse events during labor and the first 24 hours following delivery. We defined adverse events as maternal desaturation (SpO2<90%), maternal or fetal bag mask ventilation, maternal or fetal intubation, and use of maternal or fetal naloxone or epinephrine.
We compared the fentanyl PCA cohort of patients in labor (n=52), to a similar number of patients in labor (during the same time period) that did not have relative contraindications to neuraxial anesthesia, yet elected to utilize (nurse administered) intermittent 5mg IV nalbuphine boluses for pain control. It is the standard practice for obstetricians at our institution to order napbuphine 5mg as an initial dose for labor pain, followed by a second dose of nalbuphine 5mg in 3 or 4 hours as needed. We recorded the VPS prior to initiation of the fentanyl PCA or nalbuphine bolus (T=0) and at 1 hour, 2 hours, 3 hours and 4 hours.
For the purposes of our study, we considered “adherence to the protocol” as being those cases where the obstetrician initiated the fentanyl PCA with the initial recommended fentanyl doses as outlined in our protocol.
Patients’ characteristics were described using median and interquartile range (IQR) for all continuous, skewed variables, and counts and percentages for all categorical variables. Continuous variables were compared using the t-test or Mann-Whitney U test, whereas categorical variables were compared using the Pearson’s chisquare test or Fisher exact test, as appropriate. Patient verbal pain scores in the study were repeated measures data. Linear mixed-effects model (LMM) was applied to model the verbal pain score data [4]. The LMM was a generalization of the standard linear model, permitting the data to exhibit correlation and non-constant variability. It provided flexibility of modeling not only the means of the data (as in standard linear model) but also their variance and covariance. ANOVA F-type tests (Verbeke and Molenberghs, 2000) were constructed based on the mixed models to test group effect, time trend, and their interaction. All p values were reported as two tailed and a value of <0.05 was considered statistically significant. Statistical analyses were performed using SAS software (Version 9.4, SAS Institute, and Cary, NC).
Demographics of the fentanyl and nalbuphine groups can be seen in table 1. Of note, the groups differed significantly in that the fentanyl PCA group had longer first stages of labor, longer periods from initial pain medicine requests to delivery, and greater use of oxytocin injectable compared to the nalbuphine group.
| Factor | Fentanyl PCA (N=52) | Nalbupine (N=52) | p-value |
| Age | 27.0[21.0,32.0] | 27.0[22.0,30.5] | 0.72b |
| Weight | 80.8[71.0,91.8] | 84.6[67.8,102.5] | 0.45b |
| Height | 64.0[62.0,65.0] | 64.0[61.5,66.0] | 0.75b |
| BMI | 31.1[26.6,35.9] | 32.5[27.1,37.2] | 0.65b |
| Gestational Age | 38.3[36.5,39.4] | 39.1[37.3,39.5] | 0.24b |
| Cervical Dilation (T=0) | 3.0[2.0,4.0] | 4.0[2.0,5.0] | 0.074b |
| Starting Pain Score (T=0) | 8.5[7.0,10.0] | 8.5[8.0,10.0] | 0.80b |
| Length of 1st stage Labor (hours)* | 11.8[8.6,17.8] | 8.6[6.1,12.2] | 0.003b |
| T=0 until Delivery (hours) | 4.7[2.0,8.5] | 2.7[1.4,4.3] | 0.011b |
| Use of Pitocin | <0.001c | ||
| FALSE | 6(11.5) | 24(46.2) | |
| TRUE | 46(88.5) | 28(53.8) |
Table 1: Patients characteristics.
*Data not available for all subjects.
Statistics presented as Median [P25, P75] or N (column %).
p-values: b=Kruskal-Wallis test, c=Pearson's chi-square test.
There were no maternal adverse events (maternal desaturations, naloxone use, bag mask ventilation, intubation) observed in either group (Table 2). Fetal adverse events were not significantly different between the fentanyl vs nalbuphine groups for fetal bag mask ventilation (3/52 vs 4/52, p=1.0), fetal intubation (3/52 vs 3/52, p=1.0) or fetal naloxone use (1/52 vs 0/52, p=1.0). Fetal adverse events are detailed in table 3.
| Complications | Fentanyl PCA | % | Nalbuphine | % | p-value |
| (MATERNAL) | (N=52) | (N=52) | |||
| Maternal Bag Mask Ventilation | 0 | 0 | |||
| Maternal Intubation | 0 | 0 | |||
| Maternal Naloxone | 0 | 0 | |||
| Maternal Saturation <90% | 0 | 0 | |||
| (FETAL) | (N=52) | (N=52) | |||
| Fetal Bag Mask Ventilation | 3 | 0.0577 | 4 | 0.0769 | 1 |
| Fetal Intubation | 3 | 0.0577 | 3 | 0.0577 | 1 |
| Fetal Naloxone | 1 | 0.0192 | 0 | 1 | |
| Fetal Chest Compressions | 0 | 0 | |||
| Fetal Epinephrine | 0 | 0 |
Table 2: Maternal/Fetal complications.
| Fentanyl | |
| Complication | Details |
| Intubation | 1) 39 4/7 weeks gestation. Mother with history of walking corpse syndrome, gestational diabetes and preeclampsia. Refused obstetric care despite having a Category 2 tracing with frequent fetal decelerations. Underwent a Cesarean section (C/S) under spinal anesthesia for arrest of labor. Cord gas at delivery pH 6.9. Neonate had decerebrate posturing consistent with moderate encephalopathy. Neonate was intubated ~ 5 hours after neonatal intensive care unit (NICU) admission and started on cooling protocol for moderate to severe hypoxic ischemic encephalopathy. |
| 2) 37 6/7 weeks gestation. Born with meconium, found to have poor tone and respiratory effort. Intubation for 2.5 minutes when baby started spontaneous respiratory efforts. Admitted to NICU. | |
| 3) 41 weeks gestation. Born with meconium, known fetal arrhythmia and possible chorioamnionitis. Intubated immediately to aspirate the trachea, but no meconium aspirated. Baby self-extubated in NICU and was placed on nasal cannula. Intubated ~ 30 minutes. |
|
| Bag Mask Ventilation | 1)36 3/7 weeks gestation. Mother underwent a primary C/S under general anesthesia (GA) for HELLP syndrome. Bag/ mask ventilation (BMV) × 1 minute. The baby then received continuous positive airway pressure × 1.5 minutes followed by spontaneous respirations. Admitted to NICU for 2 days. |
| 2) 40 2/7 weeks gestation. Mother underwent a C/S under GA. Floppy at birth. BMV initiated. Narcan was given and baby improved in tone with good cry. Observed in NICU for 3 hours. | |
| 3) 38 3/7 weeks gestation. Spontaneous vaginal delivery. Born floppy, with no respiratory effort. Nuchal cord x 1 plus a true knot. Baby received BMV for <5 minutes. Did not require NICU admission. | |
| Nalbuphine | |
| Complication | Details |
| Bag Mask ventilation | 1) Term. Initial poor respiratory effort noted. Taken to NICU for observation. Intubation not required. |
| Bag Mask Ventilation with subsequent intubation. | 1) 22 4/7 weeks gestation |
| 2) 26 2/7 weeks gestation | |
| 3) 28 3/7 weeks gestation. Increased work of breathing noted. | |
Table 3: Fetal complications.
There were no differences in VPSs for subjects receiving fentanyl PCA where the protocol was followed, compared to those patients where the fentanyl protocol was not strictly followed (p=0.3530). Thus, all patients receiving fentanyl PCA (protocol followed and protocol not followed) were analyzed as one group (i.e. fentanyl group). The median VPS (with IQR) over time for the fentanyl and nalbuphine groups are depicted in figure 1. There was a significant difference in the VPS for the fentanyl group at 2 hours (p=0.0180) when compared to baseline. There was no difference in VPS for the fentanyl group at 3 and 4 hours compared to baseline (Table 4). There was a reduction in the VPS for the nalbuphine group at one hour (p=0.0001) and two hours (p=0.0157), but no difference at 3 and 4 hours compared to baseline (Table 2). When performing intergroup comparisons, there was a difference between the VPS for the fentanyl and nalbuphine groups at 1 hour (p=0.0012), with the nalbuphine group having a lower VPS. When comparing the two groups over the entire 4 hour study period, there was no difference between the two groups (p=0.6622).

Figure 1: Median pain scores vs time.
| Method | Time | Mean Difference Estimate | Standard Error | p-value |
| Fentanyl PCA | 1 hour vs 0 hour | -0.1526 | 0.2841 | 0.5917 |
| 2 hours vs 0 hour | -0.8279 | 0.3473 | 0.018 | |
| 3 hours vs 0 hour | 0.0441 | 0.3453 | 0.8985 | |
| 4 hours vs 0 hour | 0.1973 | 0.361 | 0.5853 | |
| Nalbuphine | 1 hour vs 0 hour | -1.7256 | 0.3013 | <0.0001 |
| 2 hours vs 0 hour | -0.8229 | 0.3378 | 0.0157 | |
| 3 hours vs 0 hour | -0.06874 | 0.3993 | 0.8635 | |
| 4 hours vs 0 hour | 0.4493 | 0.5013 | 0.3711 |
Table 4: Pain level comparison at specific time intervals based on linear mixed effects models.
Figure 2 shows the various indications for use of the fentanyl PCA during labor. The thrombocytopenia/platelet dysfunction group (n=17) could be broken down further into von Willebrand disease (2), idiopathic thrombocytopenia purpura (3), hemolysis, elevated liver enzymes and low platelets (HELLP) (4), no etiology recorded for low platelets (8). 6/52 patients were ordered the fentanyl PCA due to patient intolerance of neuraxial or patient refusal which were not one of the original indications.
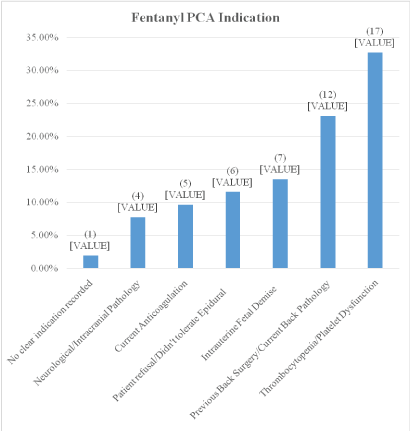
Figure 2: Fentanyl PCA indication.
Adherence to the fentanyl protocol progressively decreased with each ensuing year between 2009 and 2015: 4/5 (80%), 10/17 (59%), 5/9 (56%), 4/8 (50%), 2/6 (33%), 1/4 (25%) 0/1 (0%) respectively (Figure 3).
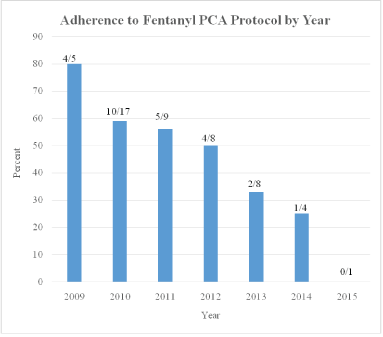
Figure 3: Adherence to protocol.
There have been numerous studies assessing the efficacy and side effects of IV PCA for labor utilizing varying opioid regimens (meperidine, fentanyl, remifentanil) [5-13]. Fetal depression and maternal respiratory depression are potential complications with opioid use to manage labor pain. However, there were no maternal complications in this series, and the fetal complications during use of our patient-controlled fentanyl protocol did not differ significantly from the complications seen when intermittent nalbuphine boluses were utilized (the routine pain regimen for patients not utilizing epidural analgesia for labor at our institution). Our study confirmed previous findings where the verbal pain score initially decreased with use of the patient-controlled fentanyl protocol, but quickly returned to baseline at 3 hours.
There has recently been considerable interest and investigations in the use of remifentanil for labor analgesia due to its pharmacodynamics profile characterized by rapid onset of action and short latency to peak effect [5,8,10-12]. The rapid hydrolysis by non-specific blood and tissue esterases to an inactive metabolite results in a short duration of action which would also be appealing to limit fetal depression. We felt our nurses and obstetricians were ill-equipped to manage airway problems or apneic episodes potentially resulting from remifentanil use. Additionally, we did not feel our unit could guarantee continuous one to one nursing care or continuous observation for patients requiring IV remifentanil PCA infusions as utilized in previous studies [5,8]. There have been case reports of respiratory arrest and cardiovascular arrest in laboring women who were receiving remifentanil PCA [14,15]. Previous studies also revealed greater maternal desaturations with IV remifentanil compared to fentanyl [8], and for these reasons, we chose fentanyl as our opiate for PCA use for labor in the unique situations where neuraxial anesthesia was relatively contraindicated.
Obstetricians at our institution rarely order or administer fentanyl for pain relief for their obstetric patients or post-surgical gynecologic patients. Our protocol recommended a dosing regimen based on previous studies [6-9,13]. While the obstetricians closely followed the recommended dosing regimen in our protocol during the early years following protocol implementation, as new residents and new faculty joined the obstetric staff, adherence to the protocol progressively declined as seen in figure 3. On occasion, we noted that obstetricians ordered very small fentanyl doses (e.g. initial fentanyl 5 mcg bolus followed by fentanyl 10 mcg maintenance boluses q 10 minutes) which were far less than recommended by our protocol, presumably resulting from their lack of familiarity with fentanyl and for fear of overdosing their patients. Others have also reported a decline in protocol adherence with time [16,17]. We believe this demonstrates the need for ongoing education following implementation of similar IV PCA protocols for labor. By reviewing our IV fentanyl protocol experience in the context of a quality initiative, we were able to add “best practice” alerts in our electronic medical record that shows the recommended dosing (and indications) when our obstetricians order IV fentanyl PCAs for labor.
Maternal sedation and respiratory compromise, as well as fetal depression are all of significant concern to practitioners when utilizing IV PCAs for labor pain relief. Previous findings related to maternal and fetal adverse outcomes during use of IV PCAs for labor have been extremely variable [5-9].
Consistent with the findings of Hosokawa [9] and Miyakoshi [7], we observed an extremely low rate of maternal and fetal complications during use of our fentanyl PCA protocol. There were no maternal adverse outcomes in our series (Table 2) and the rate of fetal naloxone use, bag mask ventilation and fetal intubation did not differ in the fentanyl PCA group compared to the intermittent bolus nalbuphine group at our institution.
Our fentanyl PCA protocol for labor was only to be used in specified situations; primarily situations where neuraxial analgesia was relatively contraindicated. When we reviewed the data from our series, we noted that fentanyl PCAs were being used in numerous situations for which it was not initially intended at our institution. Intrauterine fetal demise and patient refusal to obtain epidural analgesia were often the reasons noted for fentanyl PCA use at our institution. Given our very low rate of maternal and fetal complications, we were forced to reassess if we should “withhold” fentanyl PCA use in these situations. We still strongly maintain that all patients requesting IV fentanyl PCA use for labor must be counseled regarding the potential risks of maternal sedation and respiratory compromise, as well as risks of fetal depression and the possible need for fetal resuscitation.
Our findings for pain scores during labor while on fentanyl PCAs were consistent with earlier findings. Despite considerable doses of IV fentanyl, patients in our series continued to report very high pain scores (Figure 1). The prospective randomized study by Douma, et al. [5] evaluated the analgesic efficacy of remifentanil vs meperidine and fentanyl via PCA for labor. They assessed pain scores and sedation scores after 1, 2 and 3 hours and found that the pain scores decreased the most with remifentanil and this decrease was only significant at 1 hour. Three hours after initiation of all opioid PCAs studied, pain scores were no longer significantly different from baseline in any of the three groups. Our findings were very similar to those of Douma, et al. where there was an initial decline in pain scores in both fentanyl and nalbuphine groups, yet at 3 and 4 hours, there was no significant difference in pain scores in either of our groups when compared to baseline. We also could not detect a difference in pain scores over time when comparing the VPS for patients receiving IV fentanyl PCA vs patients receiving intermittent nalbuphine boluses. One cannot conclude that our pain regimens were ineffective simply because the pain scores increased back to baseline with time. It has been documented that pain scores increase during labor [18].
It is important to note that the fentanyl and nalbuphine groups were dissimilar in many ways. The fentanyl group had longer labors and increased use of Pitocin. The nalbuphine group did not have contraindications to neuraxial anesthesia and possibly had different expectations for their labor pain (often voluntarily opting not to receive epidurals for pain relief). This contrasts with the fentanyl PCA group, where there were often relative contraindications to neuraxial anesthesia and where many patients were quite plausibly hoping for optimal pain relief via epidural analgesia, yet their comorbidities precluded epidural use. It is further plausible that the fentanyl PCA group may have been frustrated and intolerant with their labor pain management and thus may have rated their pain higher than the nalbuphine group which may have been more accepting of their pain due to their voluntary request not to receive an epidural.
It is important to underscore that high pain scores do not necessarily indicate that patients did not find this mode of analgesia helpful and do not necessarily equate with poor patient satisfaction. Douma, et al. [5] reported satisfaction scores in the 7-8 out of 10 ranges for the three opioid groups studied despite high pain scores. The retrospective study by Miyakoshi, et al. [7] evaluated perinatal outcomes and analgesia efficacy in patients receiving IV fentanyl PCA and 72% of patients receiving fentanyl PCA rated their pain relief as excellent or good despite median pain scores of approximately 9 for most of the study period.
Our study was limited by its retrospective design as well as the strong selection bias in the two groups (with one group opting not to receive neuraxial analgesia, and the other group having this option frequently withheld due to relative contraindications to neuraxial analgesia). Due to the small number of patients utilizing fentanyl PCA for labor at our institution, the overall number of patients enrolled in our study may not have been sufficient to detect small differences in adverse events attributable to use of the fentanyl PCA protocol. However, our findings remain clinically relevant as other institutions will likely have similarly small numbers of patients using continuous opioid PCAs for labor, and these medical providers want to be reassured that significant harm has not been observed in mothers and their unborn in published series. When comparing VPS for patients at our institution following implementation of our fentanyl PCA protocol to those patients receiving intermittent nalbuphine boluses, we could not detect a significant difference in pain scores at 3 and 4 hours after initiation. We stress that one cannot however conclude that the fentanyl PCA was not effective in helping manage the patients’ pain or facilitating improved patient satisfaction as these were not specifically evaluated in our current study. Adherence to our protocol progressively decreased with time, indicating the need for ongoing educational efforts and quality initiatives. Data from our study suggests that fentanyl PCA is a safe and reasonable option for labor pain control in settings where epidural analgesia is relatively contraindicated, or simply not desired by the patient.
Download Provisional pdf here
All Sci Forschen Journals are Open Access