Article Information
Article Type: Case Report
Citation: Rogers IM (2017) Pyloric Stenosis of InfancyThe Anaesthetic Challenge. J Clin Anesth Manag 2(1): doi http://dx.doi.org/10.16966/2470-9956.124
Copyright: © 2017 Rogers IM, This is an openaccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
Received date: 20 Mar 2017
Accepted date: 18 Apr 2017
Published date: 24 Apr 2017
In 1903 Dr. Freund, an early medical observer of the extraordinary condition of pyloric stenosis of infancy (PS) declared that an excess of acid was the cause [1]. This theory was quietly forgotten over the years .and the obvious acidity of the gastric contents was presumably attributed to collection of secreted acid behind a closed pylorus. Indeed when pH only is measured there is little difference. It is only when basal acid output is measured or histamine stimulated output that a huge increase in acid secretion in babies both before and after pyloromyotomy can be detected [2,3].
In more recent years compelling evidence has accumulated that hyperacidity is indeed connected to the development of this condition-it is a primary inherited constitutional condition.
- Acid entering the duodenum is the most potent cause of pyloric sphincter contraction. [4]. It is accepted that repeated sphincter contraction leads to work hypertrophy under the influence of the trophic effects of neonatal hypergastrinaemia [5]. The mechanism of sphincter hypertrophy occurs due to the local accumulation of growth factors in response to repeated contraction facilitated by neonatal hypergastrinaemia-a known stimulant of gastrointestinal growth in the neonate. Earlier papers implied that the known accumulation of growth factors was the primary abnormality which led to sphincter hypertrophy [6]. This is clearly not so.
- Artificially stimulating acid secretion by pentagastrin injections in pregnant dogs causes sphincter hypertrophy, indistinguishable from the human variety, in 24 out of 84 puppies. 14 of the puppies had superficial duodenal ulcers [7]. The sex-ratio between males and females of 5/1 in pyloric stenosis babies (PS) parallels the sex ratio in adults with duodenal ulcer (DU) a condition classically caused by too much acid. Indeed adults with DU also share the same preponderance of Blood Group O as do PS babies. [8,9]. In addition, male premature babies are known to secrete more acid than premature females [10] a phenomenon which at a stroke would explain the sex-incidence.
- Vomiting neonates who become alkalotic have been shown always to have PS-a reflection of the highly acid nature of their loss compared to the less acid nature of the vomitus from neonates suffering from other conditions [11].
- The influence of genetics in causation is real but not exclusive. The monozygotic concordance rate while greater than that in dizygotes is still only 0.25-0.44 [12]. Differences in feeding - for example always giving what is left of the breast feed to the second twin- with the possibility of relative underfeeding - may be the explanation. Firsttime mothers with increased anxiety probably feed their vomiting baby more often, thus increasing the frequency of sphincter contraction and thus explaining the first-born predominance. It is the novice mother rather than the first-born baby which is important. The adult sphincter response to feeding is much more vigorous and much more frequent than the sphincter contractions (hunger contractions) when the stomach has emptied [13]. Hence it is relative underfeeding of the vomiting PS baby which plays an important part in the conservative management of this condition [14].
- Indeed, all the curious clinical features of PS are easily explained by the inheritance of a greater parietal cell mass than usual (inherited hyperacidity).
- The family history is consistent with the familial inheritance of an increased parietal cell mass and the male predominance on the basis of greater hyperacidity.
- Normal neonates display peak acidity at around 3 weeks of life. The rise of acidity from birth till then is thought to be due to an initial insensitivity of the negative feed-back between gastrin and antral acidity. When the negative feed- back matures at 3 weeks this peak acidity gradually falls (Figure 1) [15,16]. It is presumed that this early developmental phenomenon ensures that the acid-barrier to enteric infections is maintained until that time. Hyman et al has confirmed this peak acidity in preterm-infants. At 4 weeks of life basal acid output was 3 times greater than at 1 week with a similar increase in maximal acid outputs [17]. The regular presentation of PS at around 4 weeks has thus an easy explanation.
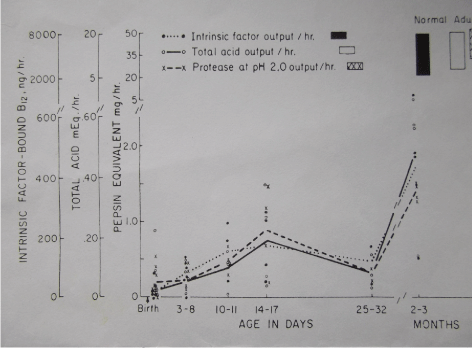
Figure 1: All parameters of gastric function acidity, intrinsic factor and pepsin peak at around 17 days
- The PS baby who survives without surgery and with or without medical treatment beyond the first few months may look forward to a complete cure. The combination of reduced acidity with a pyloric lumen which gradually widens with age is the explanation.
- Traditional medical treatment which involves variable doses of atropine with regular gastric washouts and underfeeding was originally introduced to reduce pyloric sphincter spasm. In reality all three will reduce gastric acidity and thereby reduce the frequency of sphincter contraction. The observation that neonatal erythromycin prescriptions increase the frequency of PS should come as no surprise. Erythromycin, a motilin agonist, specifically increases antral motility and causes the pyloric bulb to contract [18].
The Anaesthetic Problem
Conventional pre-operative treatment involves correcting fluid and electrolyte problems by intra-venous therapy and allowing the immature kidneys to rectify the problem. This may take 1 or 2 days.
The alkalaemia which is variable and dependant on symptom duration is found in 50 to 60% of PS babies in the Western world and poses particular problems for the anaesthetist [19,20]. Low preoperative carbon dioxide levels may lead to a reduced respiratory drive and complicate anesthetic recovery
These combined problems have led to many complicated strategies involving the volume and quality of preoperative fluid replacement [19]. All these strategies involve accepting the continual loss of acid and trying to replace and restore a normal electrolyte and acid-base balance.
There is another way. Why not immediately abolish acid, fluid and electrolyte loss at source? Why not turn the tap off?
H2 receptor antagonists have indeed been used with remarkable advantages. Banieghbal et al. [21] has shown that intra-venous cimetidine rapidly corrects the metabolic alkalosis so that same day surgery may then be undertaken. Not only do H2 receptor blockers or Proton Pump Inhibitors stop acid loss they will reduce the loss of fluid and electrolyte loss. He has also shown that when the babies with a thickness on ultrasound of 3 mm. or less are treated with intra-venous cimetidine, 16 out of 17 babies are completely cured. -but that is another story! [22].
Download Provisional pdf here


