Introduction
The metabolic control of cell hydration serves as a quantummechanical sensitive cell parameter determining the functional activity of the cell, which is realized through the surface-dependent changes of the number of functionally active membrane proteins, having enzymes [1], receptors [2] and ionic channel-forming properties [3], as well as through hydration-dependent regulation of intracellular macromolecules’ activity by folding-unfolding mechanism [4]. Therefore, the dysfunction of metabolic control of cell hydration can be considered as a common consequence of any cell pathology. However, the cell over-hydration serves as a diagnostic marker for carcinogenesis [5], while the cell dehydration-as a marker for nerve and cardiovascular disorders [6]. The data indicate that different metabolic pathways control the cell hydration in excitable and soft tissues. From the diagnostic point of view, it is important to evaluate which of these two types of tissue hydration is more sensitive to pathology of organism, including carcinogenesis.
Previously, we have shown that the cyclic nucleotide-dependent Na/Ca exchange, which has a crucial role in the regulation of intracellular Ca homeostasis (Ca2+]i) serves as a quantum-mechanical sensor through which the biological effects of extremely weak physical and chemical signals on the cells and organism are realized [7-10]. It has also been shown that the cGMP-dependent Na/Ca exchange in the forward (F) mode reverses into cAMP-dependent Na/Ca exchange in the reverse (R) mode depending on ageing. The cGMP-dependent FNa/Ca exchange can be stimulated by pM ouabain, while the cAMP-dependent RNa/Ca exchange by nM ouabain [11,12]. Although, the Na/Ca exchange functions in stoichiometry of 3Na:1Ca, its effect on cell hydration depends on the initial state of organisms: in the young animals the activation of cGMP-dependent FNa/Ca exchange leads to cell dehydration, while in the old animals the activation of cAMP-dependent RNa/ Ca exchange leads to cell hydration. So they have hydration and dehydration effects on cell, respectively [13,10].
On the basis of the mentioned age-dependent dysfunction of intracellular signaling system leading to RNa/Ca exchange-induced dehydration has been suggested as a primary cellular mechanism for the pathology of nerve and cardiovascular systems [14]. However, the role of cyclic nucleotide-dependent Na/Ca exchange in regulation of cell hydration in norm and pathology in soft tissues, including cancer tissues is not clear yet. Therefore, the aim of the present work is to evaluate the role of cAMP-dependent RNa/Ca exchange in generation of cell pathology in soft tissues. For this purpose, the comparative study of brain cortex, spleen and tumor tissue hydrations, [3H]- ouabain binding with cell membrane, the 45Ca2+ uptake by cells and intracellular cAMP contents in healthy (H) and tumor carrying (TC) mice has been performed.
Materials and Methods
Animals
All the procedures performed on animals were carried out following the protocols approved by Animal Care and Use Committee of Life Sciences International Postgraduate Educational Centre (LSIPEC, Yerevan, Armenia). Albino white male mice with the average weight of 18-20 g were used for the experiments. The experiments were performed on 300 male albino mice (150 H and 150 TC). They were regularly examined, kept under control of the veterinary in LSIPEC and reserved in a specific pathogen-free animal room under optimum conditions of 12 h light/dark cycles, at the temperature of 22 ± 2°C, with a relative humidity of 50% and were fed ad libitum on a standard lab chow and water.
Chemicals
Tyrode’s physiological solution (PS) containing (in mM) 137 NaCl, 5.4 KCl, 1.8 CaCl2, 1.05 MgCl2 , 5 C6H12O6, 11.9 NaHCO3, and 0.42 NaH2 PO4, adjusted to pH 7.4 with NaOH was used. All the chemicals were obtained from “Medisar” Industrial Chemical Importation Company (Yerevan, Armenia). The [3H]-ouabain with specific activity (25.34 Ci/mM) and non-radioactive ouabain (PerkinElmer, Massachusetts, USA) from 10-9M to 10-4M concentrations dissolved in the PS were used for intraperitoneal (i.p) injection and incubation. The volume of injected solutions was adjusted according to the weight of the animals (0.02 ml/g), the samples were incubated in 10 ml of experimental solution.
Tissue preparation
It is well known that anesthetics with different chemical and pharmacological profiles significantly affect metabolic processes, which play an important role in the regulation of cell volume [15,16]. Therefore, in the present experiments animals were sharply immobilized by freezing method (dipping their noses into liquid nitrogen for 3-4 sec) and decapitated [16]. After such a procedure the full absence of somatic reflexes on extra stimuli was recorded. For each experimental group 15 animals were chosen. The tissues of brain cortex, spleen and tumor were isolated and 3 samples from each animal were taken. The weight of each sample was from 50 to 60 mg. Thus, in each experimental group the number of tissue slices was 45.
Definition of water content of tissues
Water content of tissue was determined by traditional “tissue drying” method [17]. After measuring the wet weight (w.w.) of a tissue it was dried in oven (Factory of Medical Equipment, Odessa, Ukraine) for 24h at 105°C for determination of dry weight (d.w.). The quantity of water in 1g of d.w. tissue was counted by the following equation: (w.w-d.w.)/d.w.
Counting of [3H]-ouabain receptors in membrane
To estimate the number of Na+/K+-ATPase molecules in membrane, the number of [3H]-ouabain molecules binding with cell membrane was counted. In the in vivo experiments [3H]-ouabain was i.p injected to animals. After 30 min. animals were sacrificed and the brain cortex, spleen and tumor tissue slices were dissected and incubated in the 100 ml ouabain-free PS. To remove surface-adherent and extracellular tracer the brain cortex, spleen and tumor tissue slices were washed fivefold; each wash was about 5 min in duration, in the normal (ouabain-free) PS. After determination of wet and dry weight of samples, they were homogenized in 50 µl of 68% HNO3 solution. Then 2 ml of Bray’s scintillation fluid was added and radioactivity of samples was quantified with Wallac-1450 Liquid Scintillation and Luminescence Counter (Pribori Oy, Turku, Finland). The number of ouabain molecules binding with cell membranes was defined per mg of dry weight of samples.
Measurement of 45Ca2+ uptake
The 45Ca2+ uptake was measured by the following way: 0.0115 mM CaCl2 from 1.8 mM was substituted by radioactive 45Ca2+ (11.2 mCi/l) in the PS. Animals were i.p injected with 45Ca2+ (with 0.187 mCi/g radioactivity of body weight) dissolved in the PS. After 30 min. animals were decapitated and the brain cortex, spleen and tumor tissue samples were incubated for 30 min. in the PS (as a control) and the PS once containing 10-9M and once 10-4M ouabain. Then all the samples were dried in thermostat during 24h at 105°C. The quantity of 45Ca2+ uptake by the brain cortex, spleen and tumor tissue sample slices was expressed by cpm/mg d.w.
Determination of cAMP content in the samples
Determination of cAMP content in the samples was performed according to the cAMP [125I] RIA KIT manual (PerkinElmer Life sciences, Inc.). Precipitation of proteins from tissues has been accomplished with trichloroacetic acid (TCA). Frozen tissue samples were homogenized at 4°C with 6% TCA to make a 1 mL 10% (w/v) homogenate. Equal volume of cold 10% TCA was added supernatants. TCA extracts were centrifuged at 2,500 × g at 4°C for 15 min. The supernatant was collected and extracted 4 times with 5x volume of water-saturated ether. After this the ether phase was discarded. Later, the samples were placed in the water bath at 70-80°C and evaporated to dryness under a stream of air. The residue was dissolved in Assay Buffer and 100 µL of this solution was used directly in the Assay. Counting of the samples was performed by Wallac-1450 liquid scintillation and luminescence counter (Pribori Oy, Turku, Finland). The concentration of cAMP in the samples was determined by interpolation from the standard curve.
Statistic analysis
Microsoft Excel and Sigma-Plot (Version 8.02A, NY, and USA) were used for data analyses. Significance in comparison with the sham group was calculated with Student’s paired t-test with the following symbols (*p<0.05; **p<0.01; ***p<0.001).
Results
The comparative study of tissue hydration of H and TC mice has shown that the level of hydration in brain cortex and spleen tissues in TC mice is higher than in H mice (Table 1). It is worth to note that the differences between tissue hydrations of H and TC mice are more pronounced in spleen tissues (5.8%) than in brain cortex tissues (3.9%). Each datum is the average value of 45 pieces.
| Water content g/g d.w. |
| |
Brain cortex |
|
|
Spleen |
|
|
Ouabain Dose M |
Healthy |
Tumor carrying |
H/TC Δ2% |
Healthy |
Tumor carrying |
H/TC Δ2% |
|
0 |
4.64 |
4.82 |
3.9 |
3.45 |
3.65 |
5.8 |
| p<0.05 |
p<0.05 |
| * |
* |
|
10-4 |
5.47 |
4.17 |
23.8↓ |
3.8 |
3.45 |
9.2↓ |
| p<0.001 |
p<0.001 |
| *** |
*** |
|
Δ1% |
17.9 |
13.5↓ |
- |
10.1 |
5.5↓ |
- |
| |
p<0.001 |
p<0.001 |
p<0.05 |
| *** |
*** |
*** |
* |
Table 1: The cell hydration of brain cortex and spleen tissues in healthy (H) and tumor carrying (TC) mice in ouabain-free and 10-4M ouabain
containing physiological solution (PS).
To estimate the role of Na+/K+ pump in the increase of brain cortex and spleen tissue hydration of TC mice, the effect of 10-4M ouabain on tissue hydration as well as the [3H]-ouabain binding with cell membrane of these tissues in H and TC mice have been studied.
As the data present, the 10-4M ouabain-induced inhibition of Na+/K+ pump activity [18] increases the hydration in brain cortex and spleen tissues of H mice by 17.9% and 10.1%, respectively, and brings to dehydration by 13.5% and 5.5% in TC mice. The obtained data indicate that the Na+/K+ pump inhibition-induced hydration and dehydration of tissues in H and TC mice are more significantly expressed in brain cortex than in spleen tissues. Although, the 10-4M ouabain leads to cell dehydration in brain cortex and spleen tissues of TC mice, the [3H]-ouabain binding with cell membrane of these tissues is increased by 5.6% and 2.7% respectively, as compared with [3H]-ouabain binding with those tissues of H mice (Table 2).
| Number of ouabain molecules (*108) |
| |
Brain cortex |
|
|
|
Spleen |
|
|
Ouabain Dose M |
Healthy |
Tumor carrying |
Δ% |
Healthy |
Tumor carrying |
Δ% |
|
10-4 |
41764 |
44117 |
5.6 |
42920 |
44080 |
2.7 |
| |
p<0.05 |
| * |
* |
Table 2: The [3H]-ouabain binding with cell membrane in brain cortex and spleen tissues of healthy (H) and tumor carrying (TC) mice at presence of
10-4M ouabain in physiological solution (PS). Each datum is the average value of 45 pieces.
The next ion-transporting mechanism in membrane, which functions in electrogenic regime and is involved in the regulation of cell hydration, is Na/Ca exchange [8]. It is known that the Na+/K+ pump inactivation leads to RNa/Ca exchange as a result of [Na+]i increase [19].
Our previous study has shown that nM ouabain, which has no effect on the Na+/K pump activity stimulates RNa/Ca exchange also by the increase of intracellular contents of cAMP [20]. It indicates that nM ouabain-induced activation of cAMP-dependent RNa/Ca exchange has hydration effects on the brain cortex and heart tissues of young animals while in case of old animals it has dehydration effects on the same tissues [21]. To evaluate the role of cAMP-dependent RNa/Ca exchange in tissue hydration in TC mice compared with tissues of H mice, the protocol of previous experiments with 10-4M ouabain and [3H]-ouabain binding with cell membrane has been repeated at 10-9M ouabain concentration.
The data presented in table 3 indicate that as in case of the impact of 10-4M ouabain, the 10-9M ouabain also leads to hydration in the brain cortex and spleen tissues of H mice and dehydration in TC mice (Table 3). However, unlike the 10-4M ouabain effect, the nM ouabain-induced hydration effect is more pronounced on the spleen tissues (4.4%) than on the brain cortex tissues (3.9%), while in the tissues of TC mice the 10-9M ouabain has more pronounced dehydration effect on the brain cortex tissues (6.4%) than on the spleen tissues (4.1%).
| Water content g/g d.w. |
| Brain cortex |
Spleen |
| Ouabain Dose M |
Healthy |
Tumor carrying |
H/TC Δ2% |
Healthy |
Tumor carrying |
H/TC Δ2% |
|
0 |
4.64 |
4.82 |
3.9 |
3.45 |
3.65 |
5.8 |
| p<0.05 |
p<0.05 |
| * |
* |
|
10-9 |
4.82 |
4.51 |
6.4↓ |
3.6 |
3.5 |
2.8↓ |
| p<0.01 |
p>0.1 |
| ** |
|
|
Δ1% |
3.9 |
6.4↓ |
- |
4.4 |
4.1↓ |
- |
| p<0.05 |
p<0.001 |
p<0.001 |
p>0.1 |
| * |
*** |
*** |
|
Table 3: The effect of 10-9M ouabain on hydration in brain cortex and spleen tissues of healthy (H) and tumor carrying (TC) mice. Each datum is the
average value of 45 pieces.
The study of 10-9M, [3H]-ouabain binding with cell membrane has shown that although the 10-9M oubain in TC mice has dehydration effects on the brain cortex and spleen tissues, the number of [3H]- ouabain binding with cell membrane of the brain cortex tissues is not changed, in the spleen tissues it is dramatically increased (67%), compared with [3H]-ouabain binding with the membrane of spleen cells of H animals (Table 4).
| Number of ouabain molecules(*108) |
| Brain cortex |
Spleen |
| Ouabain Dose M |
Healthy |
Tumor carrying |
Δ% |
Healthy |
Tumor carrying |
Δ% |
|
10-9 |
5800 |
5800 |
0 |
8700 |
14500 |
66.7 |
| p<0.01 |
| ** |
Table 4: The ouabain binding with cell membrane in brain cortex and spleen tissues of healthy (H) and tumor carrying (TC) mice at presence
of 10-9M ouabain in the physiological solution (PS). Each datum is the average value of 45 pieces.
Previously it has been shown that nM ouabain in excitable cells has stimulation effect on RNa/Ca exchange [20,21]. In order to evaluate the role of RNa/Ca exchange regulation of [Ca2+]i of spleen tissues of TC mice, in the next series of experiments the study of the effects of 10-4M and 10-9M ouabain on the 45Ca2+ uptake by the brain cortex and spleen tissues in H and TC mice have been performed.
As can be seen in figure 1, in both brain cortex and spleen tissues of TC mice the 45Ca2+ uptake in ouabain-free medium is significantly higher than in H animals. The 10-9M and 10-4M ouabain have activation effects on the 45Ca2+ uptake in both brain cortex and spleen tissues, where the 10-9M effects are more pronounced than 10-4M ouabain effects (Figures 1A,1C). In the brain cortex tissues of TC mice both 10-9M and 10-4M ouabain concentrations have similar depressing effects on the 45Ca2+ uptake compared with ouabainfree medium (Figure 1B). However, in spleen cells of TC mice the ouabain sensitivity of the 45Ca2+ uptake is not significantly changed compared with the 45Ca2+ uptake in H mice (Figure 1D).
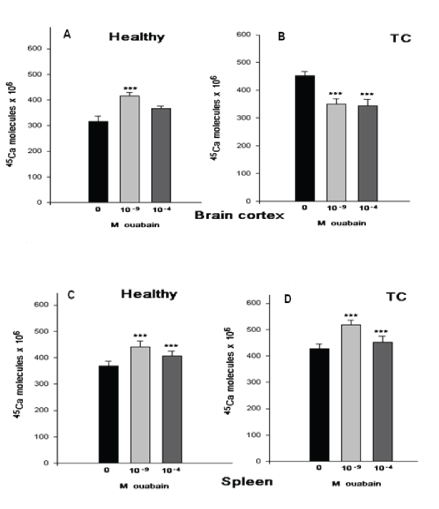
Figure 1: The 45Ca2+ uptake in in vitro experiments was determined after 30 min. incubation of ouabain-free and ouabain-containing (10-9M and 10-4M) PS in healthy (H) and tumor carrying (TC) mice. The size of columns indicates that the 45Ca2+ uptake by tissue expressed in Cpm/ mg dry weight. Under each pair of columns corresponding ouabain concentrations are noted. The 45Ca2+ uptake by brain cortex A (10-9 is 34.4% and 10-4 is 18.8%) and B (10-9 is 20% and 10-4 is 22%), spleen tissues C (10-9 is 20.5% and 10-4 is 10.3%) and D (10-9 is 24.4% and 10-4 is 6.5%) of healthy (H) and tumor carrying (TC) mice. Error bars indicate the standard error of the mean ± SEM of 45 samples. The symbols (***) indicate p<0.001, respectively. The experiments were carried out at the room temperature (22°C).
As can be seen in figure 1, in both brain cortex and spleen tissues of TC mice the 45Ca2+ uptake in ouabain-free medium is significantly higher than in H animals. The 10-9M and 10-4M ouabain have activation effects on the 45Ca2+ uptake in both brain cortex and spleen tissues, where the 10-9M effects are more pronounced than 10-4M ouabain effects (Figure 1A,1C). In the brain cortex tissues of TC mice both 10-9M and 10-4M ouabain concentrations have similar depressing effects on the 45Ca2+ uptake compared with ouabain-free medium (Figure 1B). However, in spleen cells of TC mice the ouabain sensitivity of the 45Ca2+ uptake is not significantly changed compared with the 45Ca2+ uptake in H mice (Figure 1D).
As it has been shown previously the nM ouabain-induced activation of RNa/Ca exchange in neuronal and muscle cells is realized by activation of cAMP formation, which has an age-weakening character [13,22]. To find out whether in spleen cells the activation of RNa/Ca exchange is correlated with elevation of cAMP so as in excitable cells, in the next series of experiments we have studied the intracellular cAMP contents in the brain cortex and spleen cells in H and TC mice in ouabain-free medium as well as in the PS containing 10-4M and 10-9M ouabain.
Figure 2 show that nM ouabain has elevation effect on the intracellular contents of cAMP in both brain cortex and spleen tissues of H animals, which is depressed in TC mice. But it is interesting to note that the 10-4M ouabain has no significant effect on cAMP contents in the brain cortex tissues of H mice, while in TC mice it has a slight elevation effect. In the spleen cells the 10-4M ouabain has the same activation effect on cAMP contents in both H and TC mice.
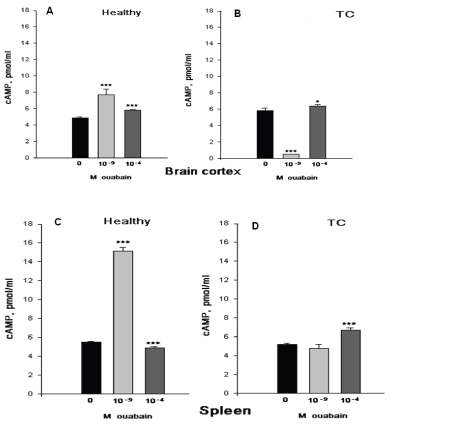
Figure 2: The effects of intraperitoneal (i.p) injection of Sham (black bars), of 10-9M (gray bars) and 10-4M (dark gray bars) ouabain on intracellular contents of cAMP in brain cortex (AB) and spleen (CD). cAMP in brain cortex A (10-9 is 66.7% and 10-4 is 27.8%) and B (10-9 is 91.3% and 10-4 is 8.7%), in spleen tissues C (10-9 is 168% and 10-4 is 9.2%) and D (10-9 is 8.3% and 10-4 is 29.2%) of healthy (H) and tumor carrying (TC) mice. Error bars indicate the standard error of the mean ± SEM of 45 samples. The symbols (*) and (***) indicate p< 0.05 and p<0.001, respectively. The experiments were carried out at the room temperature (22°C).
The presented data on the 45Ca2+ uptake and cAMP contents clearly indicate that different signaling systems control cell hydration in the brain cortex and spleen tissues in norm and pathology. The investigation of the nature of these mechanisms is the subject of our current studies.
In the next series of experiments the effects of 10-9M and 10-4M ouabain on the hydration of tissues of TC mice, the 45Ca2+ uptake and cAMP contents have been studied.
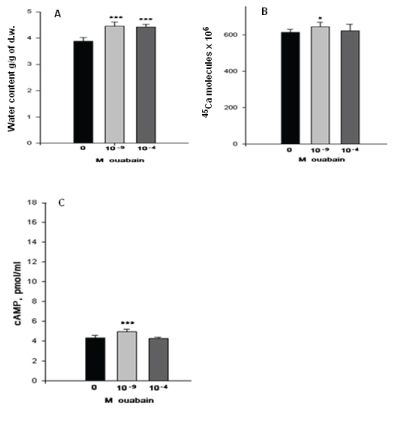
Figure 3: The effects of 10-9M and 10-4M ouabain on tumor tissue hydration (A), the 45Ca2+ uptake (B), and cAMP content (C). Figure 3A tumor tissue hydration (10-9 is 13.2% and 10-4 is 11.3%), the 45Ca2+ uptake Figure 3B (10-9 is 5.3% and 10-4 is 1.8%), and cAMP content Figure 3C (10-9 is 13.0% and 10-4 is 0.43%). Error bars indicate the standard error of the mean ± SEM of 45 samples. The symbols (*) and (***) indicate p< 0.05 and p<0.001, respectively. The experiments were carried out at the room temperature (22°C).
As figure 3A shows, both concentrations of ouabain bring to tissue hydration in TC animals. However, the stimulation effect of 10-9M ouabain on 45Ca2+ uptake is more pronounced (13.0%) than the effect of 10-4M ouabain (0.43%) (Figure 3B). It is interesting to note that in case of the 45Ca2+ uptake 10-9M ouabain there is an elevation of intracellular cAMP contents figure 3C, while 10-4M ouabain has no significant effect on it compared with ouabain-free medium.
Discussion and Conclusion
It is known that although the cell membrane is highly permeable for water, the intracellular osmotic pressure exceeds the extracellular one because of the existence of metabolic mechanism(s), which pump water from the cell. Our previous study has shown that in the membrane of neurons and myocytes the Na+/K+ pump besides controlling intracellular ionic homeostasis (cell hydration), has also depressing effects on the membrane permeability for inward ionic currents by two mechanisms: by the surface-dependent decrease of the number of functionally active ionic channels in the membrane and by the inactivation of ionic channels for inward ionic currents through the generation of net water efflux from the cells [23,3].
The Na+/K+ pump in excitable cells has a key role in generation of water efflux from the cells: it produces water efflux from the cell by generating osmotic gradients on the membrane, because of its stoichiometry of 3 Na+:2 K+, [24,25] and it, being a highly ATP utilizing machine in the membrane by stimulation of intracellular oxidative processes releases water molecules in cell cytoplasm. Thus, in the excitable cells the Na+/K+ pump have double effects on the cell hydration: on the one hand, it has dehydration effect because of its electrogenic character and properties to inhibit inward currents and on the other hand, it has hydration effect on cells by activation of intracellular oxidative process-induced release of water molecule in the cell.
It is known that unlike excitable cells, non-excitable cells have highly expressed cell volume recovery systems, which indicates the high permeability of the membrane for water [26]. Therefore, in soft tissues the Na+/K+ pump could not have significant dehydration effect on cells because of depressing electrogenic properties of the Na+/K+ pump making it unable to generate osmotic gradients on the membrane.
The data that tissue hydration is higher in TC mice than in H mice table 1 can be explained by the increase of the osmotic water uptake as a result of the impairment of metabolic water efflux from the cells or by generation of endogenous water as a result of the activation of metabolic activity of cells in response to the impact of carcinogenic factors on the cells. The evidence that 10-4M ouabain-induced pump inhibition leads to tissue dehydration in TC mice indicates the increase of metabolic nature of tissue hydration in TC mice compared with H mice. And, finally, the fact that in TC mice the spleen tissue hydration has been increased twice more than in the brain cortex tissues table 1 can be explained by the absence of dehydration effect of the Na+/K+ pump in the spleen cells.
Our earlier study has shown that cell hydration leads to the increase of ouabain receptors’ number in membrane [1]. However, the achieved results table 2 shows that in TC mice 10-4M ouabain-induced tissue dehydration is accompanied with the increase of [3H]-ouabain binding with the cell membrane compared with [3H]-ouabain binding with the cell membrane in tissues of H animals. Therefore, such opposite dynamics of ouabain-induced cell dehydration with the increase of receptor binding sites in the membrane can be explained by the increased expression of new receptors in the cell membrane of TC mice.
It is known that the inhibition of electrogenic Na+/K+ pump leads to the activation of RNa/Ca exchange, which is traditionally explained by the increase of [Na+]i [19,27]. However, our studies have shown that such an opposite correlation between Na+/K+ pump and Na/ Ca exchange is realized also by pump-induced increase of cAMP contents leading to activation of RNa/Ca [28,8]. Although, the RNa/ Ca exchange functions in stoichiometry of 3Na:1Ca it could have hydration or dehydration effect depending on initial functional activity of nerve and muscle. Our previous study has shown that nM ouabain-induced activation of RNa/Ca exchange in the brain cortex tissues of young mice has more pronounced hydration effects than 10-4M ouabain-induced activation of RNa/Ca exchange [13]. According to the literature, soft tissues are not expressed with the higher affinity isoforms (α3/α2) of Na+/K+-ATPase [29,30]. The obtained data on nM sensitivity of spleen tissue hydration and the existence of their binding sides in the cell membrane of this tissue of H mice clearly indicate that besides Na+/K+-ATPase, ouabain has other receptors in membrane the activation of which leads to stimulation of RNa/Ca exchange. The data that nM ouabain has hydration and dehydration effects on the brain cortex and spleen tissues in H and TC mice, respectively, indicate the metabolic nature, which can be due to the activation of RNa/Ca exchange. It is worth to note that in H mice the nM-induced hydration effect on spleen tissues is significantly higher (4.4%) than in brain cortex tissues (3.9%), while in TC mice the nM ouabain has more pronounced effect on the brain cortex tissues (6.4%) than on the spleen tissues (4.1%) (Table 3).
Despite the fact that nM ouabain brings to tissue hydration in H and dehydration in TC mice, the quantity of [3H]-ouabain binding with the cell membrane of brain cortex tissues of TC mice is the same as in H animals, while in the spleen tissues of TC animals it is increased by 66.67% compared with the spleen tissues of H mice. Table 4 clearly indicates over-expression of nM ouabain receptor in the brain cortex and spleen tissues of TC mice. But the over-expression of these receptors is incomparably more pronounced on spleen tissues than on brain cortex tissues. It is worth to note that since the present experiments are performed on the slices of brain cortex tissues, in which besides neurons there are also glial cells, the data that the brain cortex tissue dehydration is not accompanied with the decrease of ouabain binding with membrane can probably be due to the increase of expression of ouabain receptors in glial cells, which have high proliferative activity and are very sensitive to brain cortex pathology [31]. Based on the result of the present experiment it is difficult to conclude on the nature of newly expressed ouabain receptors in the cell membrane, therefore, special investigation is needed to evaluate it.
Our previous experiments performed on the brain cortex and heart tissues of animals have shown that in young animals the 10-9M ouabain has more pronounced activation effect on RNa/Ca exchange and tissue hydration than 10-4M ouabain effect compared with control tissues. In old animals, in case of both concentrations, ouabain has depressing effect on RNa/Ca exchange and tissue hydration [13]. Figure 2 indicates that as in case of ageing, in TC mice there is a decrease of the 45Ca2+ uptake (RNa/Ca exchange), while in the spleen cells 45Ca2+ uptake is not changed significantly.
It is known that both low and high concentrations of oubain-induced activation of Na/Ca exchange are accompanied with the intracellular cAMP contents. The nM ouabain elevates the intracellular cAMP by stimulation of G-protein in membrane [32], while 10-4M leads to the stimulation of cAMP synthesis by the increased intracellular ATP contents [28]. The obtained data in the present work point out that the brain cortex and spleen tissues of TC mice have increased the nM ouabain-sensitivity of cAMP contents, while 10-4M ouabain-sensitivity causes an increase in cAMP contents (Figure 2).
On the basis of literature evidence cAMP-induced activation of RNa/ Ca exchange of H animals leading to cell hydration can be explained by the activation of cAMP dependent Ca2+ pump in the membrane of endoplasmatic reticulum (ER) pushing Ca from cytoplasm into the ER [32], which stimulates mitochondrial activity through the zones of Mitochondria Associated Membranes’ close contact [33] and release of water molecules in cytoplasm. Therefore, the depression of nM ouabain-induced elevation effects on cAMP in brain cortex and spleen cells can be explained by losing Ca buffering properties of ER, leading to increase of [Ca2+]i in cytoplasm.
The above mentioned cases allow us to speculate that the abnormal increase of [Ca2+]i leads to cell dehydration by contraction of actin like proteins in neurons (cytoskeleton and myofibrils) and myosin in myocytes, while in soft tissues the increase of [Ca2+]i leads to cell hydration as a result of activation of lipase activity leading to increase membrane permeability for water. Our earlier work on hypotonic solution [1] as well as on breast cancer tissues [34] has shown an increased number of higher affinity ouabain receptors in membrane. Based on these data we consider the over-expression of nM-sensitive Na/Ca exchanger in TC mice as a consequence of cell hydration of spleen. This suggestion supported by the obtained results on tumor tissues figure 3 where both low and high concentrations of ouabain have approximately same hydration effect on tumor tissue although the nM ouabain stimulation effect on RNa/Ca exchange is more pronounced than 10-4M ouabain. This data can be explained by losing electrogenic effect of RNa/Ca exchange because of high permeability membrane for water. It is interesting to note that tumor tissue still enables to generate cAMP and activate RNa/Ca exchange upon the effect of nM ouabain. This phenomenon can be probably explained by the fact that still functioning of cAMP-activated in Ca pump on membrane of ER, pushing Ca from cytoplasm into ER, which in its turn stimulates RNa/Ca exchange in the cell membrane. Of course, this suggestion can be considered as a speculation and final conclusion can be done after more detailed investigation.
Thus, the obtained data in the present work brings us to the following conclusions:
- The transplantation of sarcoma-180 tumor in mice leads to the increase of hydration of the brain cortex and spleen tissues.
- Both high and low concentrations of ouabain have hydration effect on H and dehydration effects on TC mice.
- There is an increased expression of nM ouabain receptors in the cell membrane of spleen of TC mice leading to activation of RNa/ Ca exchange by the independent mechanism of the Na+/K+ pump.
- The cAMP contents in the brain cortex and spleen tissues of TC mice is dramatically decreased compared with those tissues of H animals.
- In the cell membrane of spleen tissues of TC mice there is an overexpression of Na/Ca exchangers compared with the spleen tissues of H mice.
It is suggested that over-expression of Na/Ca exchangers in soft tissues of the cell membrane could serve as a novel diagnostic marker for carcinogenesis.