Abstract
PAX8 is a cell-lineage specific transcription factor. It exerts a vital role in the development of thyroid gland, kidneys, and other derivatives of mesonephric and Müllerian ducts. Sufficient evidence advocates PAX8 as a sensitive and specific marker for thyroid, renal, and epithelial Müllerian neoplasms. Nonetheless, the current literature is scarce on PAX8 immunoreactivity, and possible diagnostic utility in non-epithelial gynecologic neoplasms. The main goal of the current study was to investigate PAX8 expression, if any in non-epithelial as well as germ cell neoplasms of gynecological origin. Accordingly, a total of 80 gynecological neoplasms were evaluated. Monoclonal PAX8 immunostain was used, and the cases were as follow; uterine leiomyoma (n=20), uterine smooth muscle tumors of uncertain malignant potential (n=2), uterine leiomyosarcoma (n=4), mature cystic teratoma (n=11), immature teratoma (n=3), struma ovarii (n=1), Sertoli-Leydig cell tumor (n=2), mixed germ cell tumors with yolk sac component (n=5), ovarian fibroma/fibrothcoma (n=16), ovarian angiosarcoma (n=1), ovarian dysgerminoma (n=2), steroid cell tumor NOS (n=1), sclerosing stromal tumor (n=1), adult (n=6) and juvenile (n=2) granulosa cell tumors, endometrial stromal sarcoma (n=1), uterine choriocarcinoma (n=1), and uterine pleomorphic undifferentiated sarcoma (n=1). Stuma ovarii revealed strong positivity, whereas yolk sac tumors were only focally positive. The remaining tumors were negative. In summary, this paper documents PAX8 focal immunoreactivity in yolk sac tumors, verification on a larger number of cases is prudent.
Keywords
PAX8; Immunohistochemistry; Müllerian; Non-epithelial; Germ cell neoplasms
Introduction
PAX8 is a cell-lineage specific transcription factor. It is one of nine members of the paired box (PAX) family of transcription factors, sharing structural similarities. PAX8 plays a vital role in the development of thyroid gland, kidneys, and organs derived from mesonephric and Müllerian ducts [1]. PAX8 is composed of 450 amino acids, and encodes a gene on chromosome 2q 13. It stains normal tissues, such as pancreatic islet cells of Langerhans, thyroid follicle cells, endometrial mucosa, ovarian inclusion cysts, epithelium of renal tubules, Bowman’s capsule and epithelium of rete testis to ejaculatory duct in males. PAX8 is reportedly negative in testicular seminiferous tubules and interstitium [2-4].
Recent evidence emphasized the utility of PAX8 immunostain -a nuclear marker- in diagnostic surgical pathology. PAX8 is expressed in thyroid, parathyroid, renal, neuroendocrine, Müllerian epithelial tumors, and B-cell lymphomas among others [5-8]. Data on PAX 8 immunoreactivity in non-epithelial tumors of gynecological origin, and germ cell tumors is lacking and/or limited. The aim of this study is to document PAX8 staining pattern in these tumors. To the best of our knowledge, this is the first reported data in the English literature in nonepithelial gynecologic tumors.
Methods
Subjects
The study was approved by Al Ain Medical District Human Research Ethics Review Committee. The protocol number is ERH-2016-428116- 013. Cerner computer based search for cases received at Tawam pathology lab was performed. The cases were identified using Cerner computer search. All gynecologic (non-epithelial and germ cell neoplasm) resections received between 2010-2015 in the pathology lab were included (i.e. consecutive). A total of 80 gynecological tumors were identified. The cases were as follow; leiomyoma (20 cases), smooth muscle tumor of uncertain malignant potential (2 cases), leiomyosarcoma (4 cases), undifferentiated pleomorphic sarcoma (1 case), choriocarcinoma (1 case), endometrial stromal sarcoma (1 case), fibroma/fibrothecoma (16 cases), dysgerminoma (2 cases), sclerosing stromal tumor of ovary (1 case), adult granulosa cell tumor (6 cases), juvenile granulosa cell tumor (2 cases), yolk sac tumor as a component of mixed germ cell tumors (5 cases), mature cystic teratoma (11 cases), struma ovarii (1 case), immature teratoma (3 cases), Sertoli-Leydig cell tumor (2 cases), steroid tumor NOS (1 case), and angiosarcoma of ovary (1 case). The original hematoxylin and eosinstained sections on all cases were independently reviewed by a pathologist (Albawardi or Alameeri) to confirm the original diagnoses.
Immunohistochemistry
PAX8 concentrated mouse monoclonal antibody from Cell Marque was used. The clone was MRQ-50 (Cat# 363m-16-Lot#1531007B, 6600 Siera College Blvd- Rocklin-USA). The positive (thyroid) and the negative (placenta, lung, and prostate) controls were placed on every slide. The validation process was performed in accordance with the College of American Pathologists requirements. The initial antibody dilution was 1:100. This was increased to 1:200 to eliminate nonspecific staining. Four microns thick sections were obtained from paraffin embedded formalin -10% buffered formalin- fixed tissue. The slides were kept in the oven for 30 min. Then deparaffinized and rehydrated. The slides were processed using low pH of 6 citrate buffer, under 97°C for 20 minutes. The 10- DAKO-48 immuno-stainer and Envision FLEX Detection Kit (polymeric method) were used. The antibody was incubated for 20 minutes, followed by mouse linker for 15 minutes, and then labelled polymer for 20 minutes, and substrate chromogen for 5 minutes repeated twice. Finally, counter stained with hematoxylin for 5 minutes. As per the antibody manufacturer’s leaflet, staining of mature B lymphocytes in tonsil, thymus, and bone marrow was reported.
Results
A total of 80 gynecologic non-epithelial and germ cell tumors were evaluated visually for PAX8 immunoreactivity. PAX8 was considered positive, if moderate or strong staining in more than 5% of tumor cell nuclei were identified [2]. Cases that did not fit this criteria were considered negative. PAX8 positive cases were further characterized as focal if the staining was patchy and involved less than 50% of tumor cell nuclei. A monoclonal mouse antibody of PAX8 was used -clone MRQ50- from Cell Marque. The manufacturer’s guide reported staining of mature B lymphocytes in tonsil, thymus, and bone marrow. In the current study, infiltrating B lymphocytes, and even plasma cells were positive. One case of struma ovarii -as expected- displayed strong and diffuse positivity for PAX8. In addition, five cases of mixed germ cell tumors revealed patchy focal moderate staining within the yolk sac component (Figure 1).
To the contrary, mature cystic teratoma (n=11), immature teratoma (n=3), sclerosing stromal tumor (n=1), Sertoli-Leydig cell tumor (n=2), ovarian stroma/follicles, ovarian fibroma/fibrothecoma (n=16), ovarian angiosarcoma (n=1), ovarian dysgerminoma (n=2), steroid cell tumor NOS (n=1), adult (n=5) and juvenile (n=2) granulosa cell tumors were all negative (Figure 2). Moreover, uterine leiomyosarcoma (n=4), smooth muscle tumors of uncertain malignant potential (n=2), and leiomyomas (n=20), including cellular variant (n=3), uterine pleomorphic undifferentiated sarcoma (n=1), endometrial stromal sarcoma (n=1), and uterine choriocarcinoma (n=1) were non-reactive (Table 1).
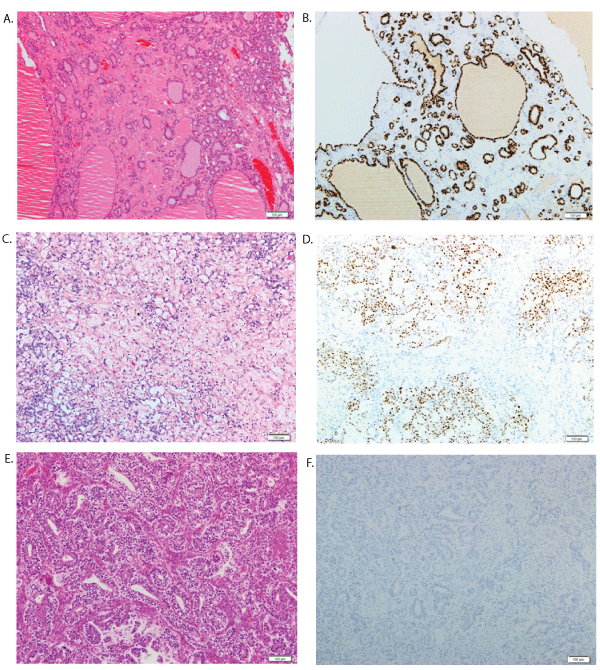
Figure 1: PAX8 expression pattern in non-epithelial and germ cell gynecologic tumors. A) Medium-power view highlight (H&E) of struma ovarii, reveal thyroid follicles filled with colloid. B) PAX8 immunostain of struma ovarii, demonstrate strong and diffuse staining of the follicular epithelium. C) Yolk sac tumor (H&E), primitive cells lining anastomosing spaces in a reticular pattern. D) PAX8 immunostain in yolk sac tumor, show focal immunoreactivity. E) Medium-power view highlight of SertoliLeydig cell tumor (H&E), Sertoli cells arrange in hollow tubules, with clusters of Leydig cells in stroma. F) Sertoli-Leydig cell tumor lack PAX8 expression.
| Histologic type |
No. of Cases |
Anatomic Site |
Negative |
Positive |
Staining Intensity‡ |
| Leiomyoma |
20 |
uterus |
20 |
___ |
___ |
| STUMP* |
2 |
uterus |
2 |
___ |
___ |
| Leiomyosarcoma |
4 |
uterus |
4 |
___ |
___ |
| Undifferentiated Pleomorphic Sarcoma |
1 |
uterus |
1 |
___ |
___ |
| Choriocarcinoma |
1 |
uterus |
1 |
___ |
___ |
| Endometrial Stromal Sarcoma |
1 |
uterus |
1 |
___ |
___ |
| Fibroma/Fibrothecoma |
16 |
ovary |
16 |
___ |
___ |
| Dysgerminoma |
2 |
ovary |
2 |
___ |
___ |
| Sclerosing Stromal Tumor |
1 |
ovary |
1 |
___ |
___ |
| Adult Granulosa Cell Tumor |
6 |
ovary |
6 |
___ |
___ |
| Juvenile Granulosa Cell Tumor |
2 |
ovary |
2 |
___ |
___ |
| Yolk Sac Tumor † |
5 |
ovary |
___ |
5 |
2+ |
| Mature Cystic Teratoma |
11 |
ovary |
11 |
___ |
___ |
| Struma Ovarii |
1 |
ovary |
___ |
1 |
3+ |
| Immature Teratoma |
3 |
ovary |
3 |
___ |
___ |
| Sertoli-Leydig Cell Tumor |
2 |
ovary |
2 |
___ |
___ |
| Steroid tumor NOS |
1 |
ovary |
1 |
___ |
___ |
| Angiosarcoma |
1 |
ovary |
1 |
___ |
___ |
Table 1: PAX8 expression in 80 gynecological non-epithelial and germ cell neoplasms.
STUMP* : Smooth muscle tumor of uncertain malignant potential.
‡ Staining intensity is scored mild (1+), intermediate (2+), or strong (3+).
† All yolk sac tumor cases were mixed germ cell tumors.
Discussion
The paired box (PAX) gene family consists of nine genes, and encodes transcription factors that governs cellular development, organogenesis, and even tumorigenesis. PAX8 is a nephritic-lineage transcription factor, essential for the development of Wolffian and Müllerian ducts [1]. The reported PAX8 immunoreactivity in B-cell lymphoma, is attributed to cross-reactivity of the N-terminal of PAX8 polyclonal antibody with the N-terminal region of PAX5 or PAX6. The latter is a recognized B-cell lineage marker. Polyclonal PAX8 and PAX5/PAX6, share high sequence homology in this region [9,10].
PAX8 has a limited expression in adult tissue. It is a specific marker for renal and Müllerian tumors [11]. The medical literature emphasized the utility of PAX8 as a sensitive marker for renal cell, ovarian epithelial, thyroid and endometrial carcinomas [2]. For example, PAX8 is expressed in 44-71% of ovarian surface epithelial cells [12], and is a useful marker in the diagnosis of renal cell carcinoma staining 83% of the cases. In fact, PAX8 stains both primary and metastatic renal tumors [12,13]. A limited number of male genital tract epithelial tumors showed PAX8 immunopositivity. PAX8 immunoreactivity was observed in serous cystadenoma of epididymis, carcinoma of rete testis, Wolffian adnexal tumor and endometrioid carcinoma of seminal vesicle [3]. Breast, pancreaticobiliary, and gastrointestinal neoplasms are negative for PAX8, which makes it a valuable marker in the diagnostic panel of metastatic carcinoma with unknown primary [2].
On the other hand, the current data on PAX8 staining pattern in nonepithelial neoplasms is scarce. One study reported PAX8 being negative in uterine adenomatoid tumors [14]. Cases of peritoneal mesothelioma were negative except for one type A case [15].Another study, documented lack of PAX8 staining in melanoma, gastrointestinal stromal tumors, leiomyosarcoma, and pheochromocytoma [2]. Six cases of endometrial stromal sarcoma with urinary bladder involvement, demonstrated PAX8 reactivity in one case [16]. Roma [17] documented PAX8 reactivity in two cases of mesonephric carcinosarcoma. He noted that the staining intensity was weaker in the sarcoma component. Two cases of ovarian yolk sac tumor (YST), were reportedly negative for PAX8 [18]. A hundred testicular germ cell tumors were studied using tissue microarrays, failed to document PAX8 immunoreactivity using polyclonal antibody [19]. A study from MD Anderson Cancer Center, identified 32 cases of primary mediastinal seminomas, and described PAX8 expression in 2 (6%) of their cases [20].
Uterine carcinosarcoma, showed variable PAX8 expression between different tumor components i.e. carcinomatous, sarcomatous, and undifferentiated, the reported PAX8 positivity was 97%, 73%, and 33% consecutively [21].
In this study, most of the evaluated gynecological neoplasms were negative for PAX8 monoclonal antibody. The list include; ovarian fibroma/fibrothecoma, dysgerminoma, granulosa cell tumors, ovarian angiosarcoma, uterine leiomyoma, uterine smooth muscle tumor of uncertain malignant potential, endometrial stromal tumors, uterine undifferentiated sarcoma, and choriocarcinoma. One case of struma ovarii demonstrated intense PAX8 staining (Figure 1). In addition, yolk sac tumors revealed focal moderate PAX8 staining (Figure 2). This last finding, contrast with most of the reported data in the English literature [18]. However, Ravishankar et al. [22], observed variable expression of PAX8 immunostain in yolk sac tumors. The inconsistency in PAX8 staining of tumors may be attributed to the use of different clones, and/ or methodology. Another source of variability stems from the visual assessment and interpretation of immunostain(s) reactivity in daily clinical practice. The visual assessment is to a certain extent subjective, and may introduce heterogeneity in results. It should be noted that visual assessment was used in scoring PAX8 immunoreactivity.
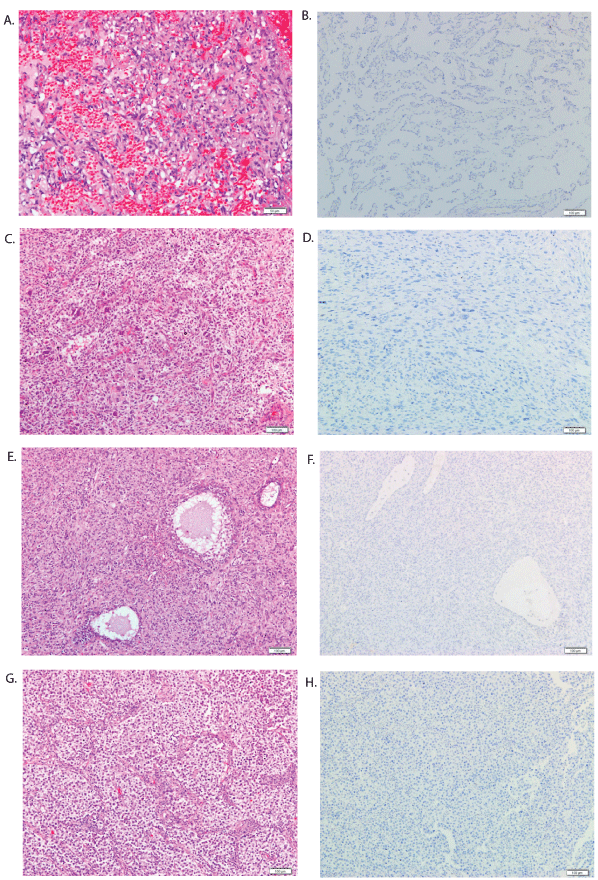
Figure 2: PAX8 immunohistochemical staining of non-epithelial and germ cell gynecologic tumors. A) Medium-power view (H&E) of angiosarcoma, reveal an anastomosing vascular channels lined by atypical endothelial cells. B) Angiosarcoma show no immunoreactivity to PAX8. C and D) Medium-power view highlight demonstrate undifferentiated pleomorphic sarcoma negative for PAX8. E) Juvenile granulosa cell tumor (H&E), demonstrate sheets of tumor cells interrupted by follicles of variable size and shape. F) Juvenile granulosa cell tumor negative for PAX8. G and H) Medium-power view highlight of dysgerminoma and the characteristic alveolar pattern with intersecting fibrous septa, the tumor is negative for PAX8.
Yolk sac tumor PAX8 immunoreactivity is of particular diagnostic interest, especially when juvenile granulosa cell tumor or embryonal carcinoma are in the differential diagnosis, both being negative for the stain. PAX8 staining in yolk sac tumors –particularly papillary and reticular patterns- may create a diagnostic pitfall with papillary renal cell carcinoma or even renal tubulocystic carcinoma. Therefore, awareness of the clinical history and the radiological findings, as well as careful microscopic examination for additional features are of paramount importance [23].As such, the presence of foamy histiocytes in fibrovascular cores pinpoints towards papillary renal cell carcinoma, while hobnail nuclei should suggest the possibility of renal tubulocystic carcinoma. A suitable panel of immunostains should be employed whenever in doubt (e.g. CD10, AMACR).
The cross-reactivity with polyclonal PAX8 antibodies is widely observed, and is related to the high sequence homology with PAX5 and PAX6, both are immuno-markers of B cells from pro-B to plasma cell stage. In the current study, a monoclonal mouse antibody (clone MRQ50) from Cell Marque was used. The infiltrating B lymphocytes, and even plasma cells were positive. Cross reactivity with monoclonal antibodies has not been reported. However, as per the manufacturer’s - Cell Marque – brochure, staining of mature B lymphocytes in tonsil, thymus, and bone marrow is known. Nonetheless, the stain was repeated doubling the original antibody dilution with similar results. This calls for caution in the interpretation of the monoclonal antibody being used. Familiarity with the specifics of the particular clone in use is necessary. In theory, monoclonal antibodies should not stain B lymphocytes [24,25]. There is no reported cases in the literature yet that suggests cross reaction. PAX8 immunostain is remains a valuable marker, in investigating advanced pelvic and/or abdominal malignancies of unknown origin. Peculiar cross reactivity even with monoclonal antibodies, and focal staining yolk sac tumors mandates extra caution.
Conclusions
In summary, this study investigated PAX8 expression in a limited number of gynecological neoplasms non-epithelial and germ cell. Of note is the focal positivity in yolk sac tumors. A finding that is worth studying on a larger number of tumors, and preferably using an objective tool of scoring immunoreactivity such as computer analogue-reader. This will enhance current knowledge on PAX8 technical difficulties and staining pattern(s).
Acknowledgement
We acknowledge the UAE University for funding the study (startup grant # 31M116).
Conflict of interest
The authors declare that they have no conflict of interest.



