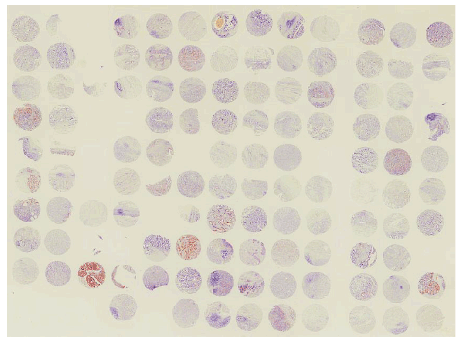
Figure 1: TRPS1 staining in the tissue microarray.
Okumura T1* Kojima H1 Hashimoto I1 Watanabe T1 Shibuya K1 Hojo S1 Yoshioka I1 Matsui K1 Nagata T1 Shimada Y2 Tsukada K 1
1Department of Surgery and Science, Graduate School of Medicine and Pharmaceutical Sciences, University of Toyama, Toyama, Japan*Corresponding author: Okumura T, MD, PhD, Department of Surgery and Science, Graduate School of Medicine & Pharmaceutical Sciences, University of Toyama, 2630 Sugitani, Toyama City, Toyama, 930-0194 Japan, Tel: +81-76-434-7331; Fax: +81-76-434-5043; E-mail: okumura@med.u-toyama.ac.jp
Article Type: Research Article
Citation: Okumura T, Kojima H, Hashimoto I, Watanabe T, Shibuya K, et al. (2016) Loss of TrichoRhino-Phalangeal syndrome-1 (TRPS1) expression as a Biomarker of Poor Prognosis in Patients with Gastric Cancer. Int J Cancer Res Mol Mech 2(2): doi http://dx.doi.org/10.16966/2381-3318.124
Copyright: © 2016 Okumura T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
Background: Tricho-rhino-phalangeal syndrome-1 (TRPS1), a multi zinc-finger nuclear protein, has been reported to regulate cell proliferation, epithelial-to-mesenchymal transition (EMT), differentiation and apoptosis during development and tumor progression. The aim of this study was to investigate clinico-pathological significance of TRPS1 expression in gastric cancer (GC).
Materials and Methods: The expression of TRPS1 was detected by immunohistochemistry in 110 surgically resected GC specimens using a tissue microarray (TMA). The patients consisted of 78 males and 32 females. Average age was 68.4 years old. The TNM stages of the patients were as follows: stage I, 17 patients; stage II, 34; stage III, 36; and stage IV, 23. After careful examination by two independent researchers, the immunohistochemical results for these patients were scored according to intensity and distribution. We additionally obtained surgically removed specimens from 10 gastric cancer patients to immunohistochemically detect the expression of TRPS-1, as well as to extracted RNA to detect the expression of microRNA (miR) such as miR221 and miR222.
Results: Among the 110 patients with GC, 30 patients (27.3%) and 80 patients (72.7%) showed high and low expression of TRPS1, respectively. Low expression of TRPS1 was associated with distant metastasis (p=0.01). Age, gender, depth of the tumor, lymph node metastasis, TNM stage and histological grade were not associated with TRPS1 expression. The overall and cause-specific survivals of the TRPS1-low patients were significantly worse than those of the TRPS1-high patients (Wilcoxon p=0.043 and p=0.048, respectively). However, TRPS1 expression was not an independent prognostic factor of the patients. In the 10 surgically resected gastric cancer tissues, the lower expression of TRPS1 was significantly associated with higher expression of miR221 (p=0.017) and miR222 (p=0.007).
Conclusion: Down regulation of TRPS1 expression was associated with distant metastasis and was an unfavorable prognostic factor in patients with GC, providing us with a novel prognostic marker and a potential therapeutic target. miR221 and miR222 were suggested to have a role in regulation of TRPS1 expression in GC.
Gastric cancer; TRPS1; Prognostic marker; Immunohistochemistry; miR221/222
Gastric cancer (GC) is one of the most common malignancies with increasing incidence worldwide [1]. Despite improvement in surgical outcomes and chemotherapeutic drugs, GC remains the second leading cause of cancer-related death worldwide [2]. Development of GC is associated with a number of molecular abnormalities, including the inactivation of various tumor suppressor genes and/or activation of various oncogenes [3,4]. Therefore investigations into molecular mechanism involved in malignant feature of gastric cancer are needed to establish novel biomarkers for the early detection and/or the prediction of its prognosis.
Tricho-rhino-phalangeal syndrome-1 (TRPS1), a multi zinc-finger nuclear protein, is implicated in trichorhinophalangeal syndrome (TRPS), a genetic disorder characterized by short stature, cone-shaped ends of the long bones, and distinctive facial features linked to skeletal abnormalities [5-7]. TRPS1 regulates cell proliferation, epithelial-to-mesenchymal transition (EMT), differentiation and apoptosis essentially in bone and cartilage during development [8-13].
In addition, recent reports demonstrated significant roles of TRPS1 in human cancers, such as prostate [14,15], colon [16], endometrial [17] and breast cancer [18-21]. TRPS1 has been reported to be down-regulated by microRNAs (miR) such as miR221 and miR222, and negatively regulate the EMT [22] in breast cancer. However, its roles in gastric cancer have not yet been investigated.
In this study, we detected the expression of TRPS1 in gastric cancer by immunohistochemistry using a tissue microarray (TMA) to analyze its correlation with clinic-pathological characteristics and patient’s prognosis. Then we detected the expression of miR221 and miR222, to assess the relationship between their expression and TRPS-1.
Among the 130 consecutive patients who underwent gastrectomy due to primary GC in our hospital from January 2001 to June 2006, 110 cases were evaluated because some spots in the TMA did not have enough cancer cells for evaluation or they peeled off during the staining procedure. There were 78 male and 32 female. The average age of the patients was 68.4 years. TNM stages (version 6) of the patients were as follows: stage I, 17 patients; stage II, 34; stage III, 36; and stage IV, 23. The stage IV cases had distant lymph node metastases (M1 Lymph) or single liver metastasis (H1) which has been surgically resected without residual tumor. Nineteen and 21 cases received preoperative and postoperative cisplatin-based chemotherapy, respectively. The median follow up period was 49 months ranging from 1 to 151 months.
In addition, we obtained enough size of frozen specimens of the resected tumor and corresponding normal gastric mucosa to extract RNA, and also the formalin-fixed paraffin-embedded (FFPE) samples to perform immunohistochemical staining from 10 patients who underwent gastrectomy due to primary GC in our hospital between January 2014 and January 2015. There were 5 male and 5 female. The average age of the patients was 72.6 years. TNM stages (stage II/III) and histology (Intestinal type/Diffuse type) of the patients were 4/6 and 4/6, respectively. None of the patients received preoperative chemotherapy.
This study was approved by the institutional review board at the University of Toyama (#20-57).
The tumor areas were selected with matched hematoxylin and eosin (HE)-stained slides and marked directly on the donor block. Each cylindrical tissue sample was cored (0.6 mm in diameter) from selected regions in the donor block and extruded directly into the recipient block. Multiple 4 lm sections were cut with a microtome and transferred to glass slides (Superfrost Plus, Fisher Scientific GmbH, Schwerte, Germany) [23].
A rabbit polyclonal anti-TRPS1 antibody (dilution 1:100, Abcam Inc., Cambridge, MA) was used for the immunohistochemical staining. Glass slides with the primary antibodies were incubated for 30 min in room temperature. After washing three times with KN buffer (KN-09002: Pathology Institute, Toyama, Japan), the slides were incubated for 30 min with ChemMate ENVISION K5027 (Dako, Glostrup, Denmark) and washed three times in KN buffer, and then incubated for 10 min with DAB (Dako). After the development, the slides were washed twice with distilled water, lightly counterstained with Mayer’s hematoxylin, dehydrated, cleared, and mounted with a resinous mounting medium. All procedures were carried out at room temperature.
Two researchers analyzed the expression of TRPS1 independently and scored the intensity of expression as 0 (no expression), 1 (weak expression), 2 (moderate expression), or 3 (strong expression). They also scored the distribution of expression as 0 (none), 1 (1-50% of tumor cells), or 2 (51-100% of tumor cells). TRPS1 expression (TRPS-1 IHC Score) was evaluated based on the sum total of the staining intensity and distribution.
On the basis of the total score, each patient was then classified into one of two groups, which were a low expression group (lower group: total score of 0-2) or a high expression group (higher/upper group: total score of 3-5) [23,24].
Total RNA was extracted from frozen sections of surgically removed GC tissues and corresponding normal gastric mucosa using the TRIzol Reagent (Invitrogen, Carlsbad, CA, USA) according to a standard protocol. cDNA was prepared from total RNA samples using the Taq Man microRNA reverse transcription kit on the ABI Prism 7000 realtime PCR system, according to the manufacturer’s instructions (Applied Biosystems). Predesigned Taq Man microRNA assays for hsa-miR-221 (Assay ID000524), hsa-miR-222 (Assay ID002276) and RNU6B (Assay ID 001093) were purchased from Applied Biosystems. qRT-PCR was performed using a Taq Man universal PCR master mix, according to the manufacturer’s protocol (Applied Biosystems). The microRNA quantities were analyzed in duplicate and normalized against U6B as an internal control. The average expression level (T/N ratio) of each miRNA in the 10 GC cases was calculated and log2-transformed.
Statistical analyses were performed using JMP v.11 (SAS Institute Inc., Cary, NC, USA). The chi square test, Fisher’s exact test, and the t-test were used to compare clinicopathological data. The overall survival (OS) rate after surgery was calculated for each group by the Kaplan–Meier method, and differences were assessed by the Wilcoxon test. Differences in microRNA expression levels between two variables were analyzed by the Student’s t-test. A p-values of <0.05 was considered to be statistically significant.
The expression of TRPS1 was assessed by immunohistochemical staining using a tissue microarray (Figure1). In the 110 cases which were appropriately evaluated, TRPS1 was expressed in both the membrane and cytoplasm, but mainly in the cytoplasm of the tumor cells with various intensities (Figures 2A and 2B). Among the 110 patients, 30 patients (27.3%) were TRPS1 high and 80 patients (72.7%) were TRPS1 low. Low expression of TRPS1 was associated with distant metastasis (p=0.01). While, age, gender, depth of the tumor, lymph node metastasis, TNM stage and histological grade were not associated with TRPS1 expression (Table 1).

Figure 1: TRPS1 staining in the tissue microarray.
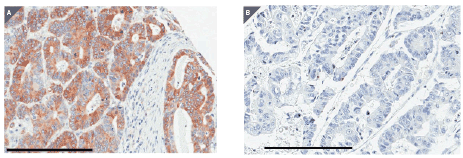
Figure 2: Representative staining for TRPS1 in tumor sections from the tissue microarray. (A) A tumor spot with high expression of TRPS1. (B) A tumor spot with low expression of TRPS1. Black bars indicate 200 micron.
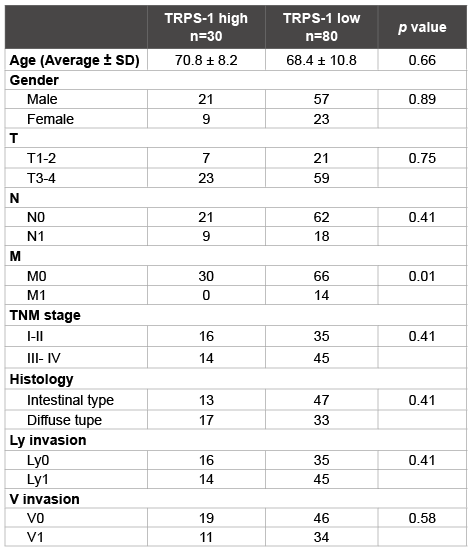
Table 1: Correlation between TRPS1 expression and clinicopathological
characteristics
TNM stage, version 6
The overall and cause-specific survivals of the TRPS1-low patients were significantly worse than those of the TRPS1-high patients (Wilcoxon p=0.043 and p=0.048, respectively; Figure 3). However, TRPS1 expression was not an independent prognostic factor of the patients (Table 2).
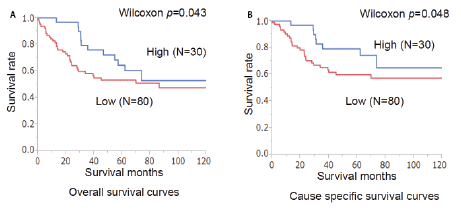
Figure 3: Overall (A) and cause-specific (B) survival curves of the patients in relation to the expression of TRPS1. The prognosis of the patients with a low expression of TRPS1 was significantly worse than that of those with a high expression of TRPS1 (p=0.043 and p=0.048, respectively).
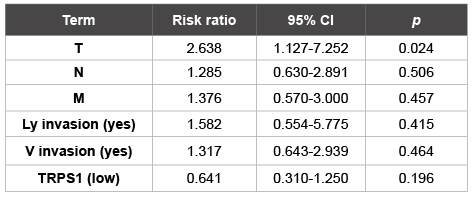
Table 2: Cox multivariate analysis of the patient data
Compared to the corresponding normal gastric mucosa, the expression of miR221 and miR222 were up regulated more than a 0.5 Log2-fold change in 6 of 10 and 5 of 10 examined cases, respectively (Figure 4A). TRPS-1 was expressed in 2 of 10 examined cases with various intensities (Figures 5A and 5B). The up regulation of miR221 was associated with lower expression of TRPS1 (p=0.017, Table 3). The up regulation of miR222 was associated with lower expression of TRPS1 (p=0.007, Table 4) as well.
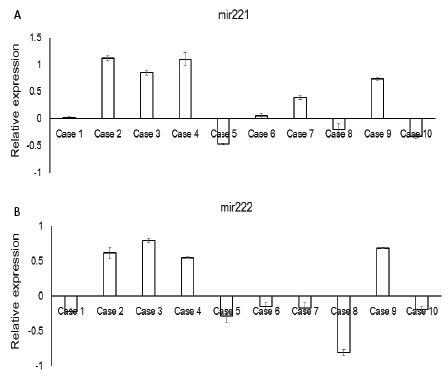
Figure 4: The expression of miR221 and miR222 in the frozen specimens of surgically resected gastric cancer cases. (A) The expression of miR221. (B) The expression of miR222. The average expression level (T/N ratio) of each miRNA was calculated and log2-transformed.

Figure 5: Representative staining for TRPS1 in tumor sections in which the expression of miR221/222 were examined. (A) A tumor (Case 8) with high expression of TRPS1. (B) A tumor (Case 3) with low expression of TRPS1. Black bars indicate 100 micron.
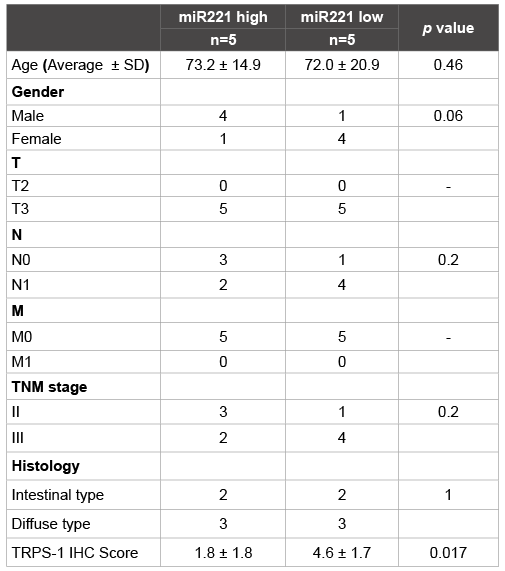
Table 3: Correlation between miR221 expression and TRPS1 expression in surgically resected specimens
Recent reports demonstrated a central role of TPRS1 in the regulatory network controlling the cell cycle and tumorigenesis in various type of cancer [25], however, its role in gastric cancer has not yet been elucidated.
Our immunohistochemical investigation of surgical gastric cancer specimens revealed that the low expression of TRPS1 was associated with distant metastasis. The survivals of the TRPS1-low patients were significantly worse than those of the TRPS1-high patients as well, suggesting that down regulation of TRPS1 was involved in the progression and metastasis of gastric cancer.
Our results were compatible with recent reports that TRPS1 negatively regulates the EMT [22] and controls cell cycle progression and cell proliferation [25] in breast cancer.
On the other hand, the increased expression of TRPS1 was reported to be involved in the pathogenesis and to be an independent poor prognostic factor in colon cancer [16], suggesting that a diverse molecular response is mediated by TRPS1 in different types of cancer.
More recent work demonstrates that miR-221/222 targets TRPS1 to induce EMT in breast cancer [26] and that TRPS1 down-regulation by miRNA-221 is essential for platelet-derived growth factor (PDGF)- mediated EMT in pancreatic cancer cells [27]. In gastric cancer, high expression of miR-221 was reported to have a significant correlation with advanced tumor-node-metastasis stage, local invasion and lymphatic metastasis [28]. Overexpression of miR-221 was also reported to be an unfavorable prognostic factor in patients with gastric cancer [28]. Another report demonstrated that miR-221 and miR-222 regulate cell growth and invasion of a gastric cancer cell line [29].
Taken together with our present results that the lower expression of TRPS-1 was significantly associated with higher expression of miR221 and miR222, it is suggested that miR221/222 targets TRPS1 to induce malignant phenotype in gastric cancer as well. Transfection of gastric cancer cell lines with miR221/222 expression vector to assess the down regulation of TRPS1 along with progression of malignant phenotype may provide us with the basis of the prognostic significance of TRPS1 and a novel target for therapy.
In conclusion, our results suggest that down regulation of TRPS1 expression is associated with distant metastasis and is an unfavorable prognostic factor in patients with gastric cancer, providing us with a novel prognostic marker and a potential therapeutic target.
This study was supported by a Grant-in-Aid for Scientific Research (C) from MEXT KAKENHI.
Download Provisional pdf here
All Sci Forschen Journals are Open Access