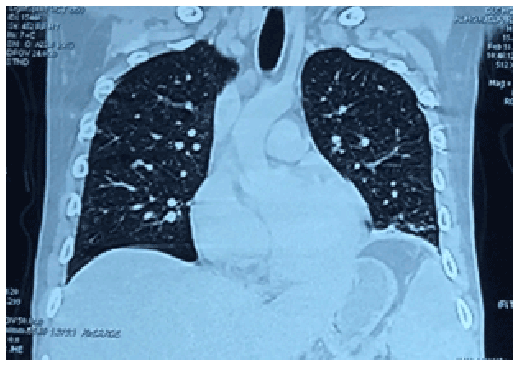
Figure 1: Frontal view of the contrast-enhanced computed tomography (CT) scan of the chest showing multiple tiny, non-calcified pulmonary parenchymal nodules on both right and left lung fields with ground-glass opacities scattered throughout.
Uy AB* Garcia AM Manguba A Loyola A
Department of Medicine, University of the Philippines- Philippine, Manila, Philippines*Corresponding author: Uy AB, Department of Medicine, Philippine General Hospital, Taft Avenue, Metro Manila, Philippines, E-mail: beauy_md@icloud.com
Aritcle Type: Case Report
Citation: Uy AB, Garcia AM, Manguba A, Loyola A (2016) Tuberculosis: the Great Lymphoma Pretender. Int J Cancer Res Mol Mech 2(1): doi http://dx.doi. org/10.16966/2381-3318.123
Copyright: © 2016 Uy AB, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
Lymphoma and tuberculosis (TB) share common features, thus misdiagnoses are commonly encountered. The Philippines is ninth out of the 22 countries in the world with the highest TB-burden, while lymphoma has age-standardized rates of 4.6/100,000 population in all ages in Manila. This study reports a case of a 24-year-old HIV-negative male presenting with a six-month course of anorexia, weight loss, fever, night sweats, and hepatosplenomegaly. Heamatologic studies revealed anemia. Bilirubins, alkaline phosphatase, and lactate dehydrogenase were elevated, while transaminases were normal. Initial chest X-ray showed nodular infiltrates on both lung apices and a negative sputum acid-fast bacilli smear. Contrast-enhanced computed tomography (CT) scan of the chest and abdomen revealed multiple tiny, non-calcified pulmonary parenchymal nodules on both lung fields, marked hepatomegaly with multiple nodules in the portahepatic, retroperitoneal and mesenteric region consistent with lymphadenopathies; and splenomegaly with intraparenchymal cystic nodules. Primary considerations were gastrointestinal lymphoma versus TB. The patient was diagnosed late with severe disseminated TB affecting the lungs, liver, spleen, genitourinary tract and intestines. No anti-TB treatment was started within the patient’s six-month course because of uncertainty in the diagnosis, especially in light of the negative sputum acid-fast bacilli smears. Malnutrition, anemia, hypoalbuminemia, and an overall immune compromised state contributed to the demise of the patient while undergoing surgery for a ruptured viscus from ileocecal TB invasion. TB is a disease that can be easily controlled and treated. The decision to start empiric treatment with anti-TB medications has a higher benefit versus risk of complications, especially in a patient with a high index of suspicion for the disease.
Tuberculosis; Lymphoma
Lymphoma is a malignancy of the lymphoid tissue, while tuberculosis (TB) is an infection by Mycobacterium tuberculosis primarily affecting the respiratory system but can also affect many different organ systems including the lymph nodes, hepatobiliary system, and gastrointestinal (GI) tract to name a few. Lymphoma and TB are commonly being misdiagnosed due to the similarities in presentation of constitutional symptoms, subjecting patients to incorrect treatment and potential harm. In the Philippines, TB is the sixth leading cause of morbidity and mortality; the country is ninth out of the 22 countries in the world with the highest TB-burden [1-3]. On the other hand, lymphoma has age-standardised rates of 4.6 per 100,000 population in all ages in Manila and Rizal [4].
The authors present a case of a 24-year-old male with a six-month history of anorexia, weight loss, low-grade fever, night sweats and generalised weakness, with intermittent and progressive piercing upper abdominal pain that was not associated with food intake or night-time awakening. He also complained of abdominal fullness, early satiety, and bone pains. The patient denied cough, hemoptysis, chest pain, breathlessness and bowel movement changes, melena, and hematochezia. The patient was an occasional alcoholic beverage drinker and occasional cannabis user. Sexual history revealed unprotected intercourse with nonpromiscuous heterosexual partners. There was no history of previous exposure or treatment for TB, radiation, and chemicals; and there was no family history of any malignancy.
Upon admission, the patient appeared cachectic (body mass index=15 kilograms per meters squared) with temporal and extremity muscle wasting. He was haemodynamically stable and had intact sensorium. He had icteric sclerae and pale palpebral conjunctivae, with no palpable peripheral lymphadenopathies. Breath sounds were clear but decreased on bibasilar lung fields. The abdomen was enlarged with normoactive bowel sounds. The liver span is 16 cm at the right midclavicular line, and Traube’s space was obliterated. Direct tenderness of the right and left quadrants of the abdomen was elicited, but with no rebound tenderness. The abdomen was dull to percussion, but with no fluid wave. Digital rectal exam was unremarkable.
Initial work-up done included a chest X-ray showing nodular infiltrates on both lung apices and a negative sputum acid-fast bacilli (AFB) smears taken serially. Contrast-enhanced computed tomography (CT) scan of the chest and abdomen (Figures 1-3) revealed multiple tiny, non-calcified pulmonary parenchymal nodules on both right and left lung fields with ground-glass opacities scattered throughout; marked hepatomegaly with multiple nodules in the portahepatic and retroperitoneal regions as well as in the mesenteric region consistent with lymphadenopathies; splenomegaly with multiple intraparenchymal cystic nodules, and thickened bowel wall. No mediastinal, hilar or axillary lymphadenopathies were noted.
Other work-ups revealed a normocytic, hypochromic anemia, which may be attributed to chronic disease. Peripheral blood smear showed toxic granulations, anisocytosis, giant platelets and basophilic stippling. Bilirubins, alkaline phosphatase, and lactate dehydrogenase (LDH) were elevated, while transaminases were normal. Aspiration biopsy of the largest portahepatic nodule came back positive for acid-fast bacilli. Human immunodeficiency virus (HIV) antibody-based test and western blot was non-reactive.

Figure 1: Frontal view of the contrast-enhanced computed tomography (CT) scan of the chest showing multiple tiny, non-calcified pulmonary parenchymal nodules on both right and left lung fields with ground-glass opacities scattered throughout.
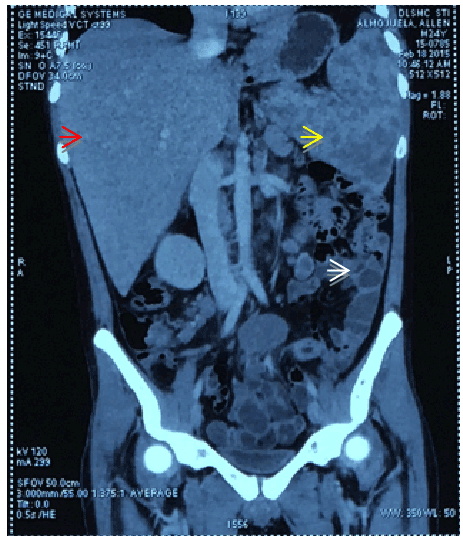
Figure 2: Frontal cut from the contrast-enhanced abdominal CT scan showing hepatomegaly with multiple intraparenchymal nodules (red arrow) in the portahepatic and retroperitoneal regions as well as in the mesenteric region consistent with lymphadenopathies, splenomegaly with multiple intraparenchymal cystic nodules (yellow arrow), bowel wall thickening as indicated by white arrow.
Lymphoma versus disseminated TB involving the lungs, liver, spleen, and GI tract were primarily considered, while keeping in mind the possibility of the simultaneous occurrence of these two clinical entities. Other differential diagnoses include systemic candidiasis, mycobacteria other than TB, Kaposi’s sarcoma, and disseminated cryptococcosis.
While undergoing work-up, the patient presented with an acute abdomen, which was detected on an upright chest X-ray (Figure 4) showing free intraperitoneal air, clinically accompanied by persistent abdominal pain. The findings were compatible with a diagnosis of peritonitis secondary to small bowel perforation; hence, an emergency exploratory laparotomy was performed.
Intraoperative findings revealed an enlarged liver and spleen, two liters of ascitic fluid, enlarged mesenteric lymph nodes, contracted small bowel mesentery, and three-point perforations of the small bowel. Macroscopically, the ileum and caecum showed tan-brown serosa with multiple cream white patchy areas. Ziehl-Neelsen staining was positive for M. tuberculosis from samples in the ileocecum. TB polymerase chain reaction (PCR) also revealed high levels of M. tuberculosis. The patient died shortly from multi organ dysfunction syndrome while undergoing surgery.
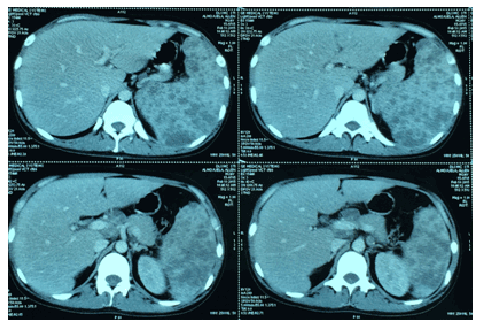
Figure 3: Sagittal cuts from the contrast-enhanced abdominal CT scan showing the multiple intraparenchymal nodules in the portahepatic (hepatic region) and splenic regions (yellow arrow).
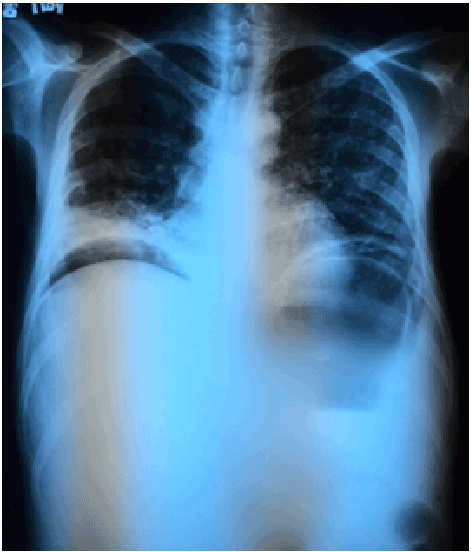
Figure 4: Chest radiograph showing diffuse reticulonodular infiltrates (white arrow) suggestive of pulmonary TB and free intraperitoneal air from a ruptured viscus (red arrow).

Figure 5: ection of the ileum showing Langhans type giant cells (magnified) and caseation necrosis, compatible with a chronic granulomatous ileitis of tuberculous etiology.
Disseminated TB is characterised by clinical features suggestive of TB involving at least two non-contiguous sites resulting from lymphohematogenous dissemination of M. tuberculosis. The lymph nodes are the most common site of extrapulmonary TB; abdominal TB comes in as sixth [5-7]. On the other hand, the GI tract is the predominant site of extranodal lymphoma, with the non-Hodgkin type (NHL) being the most common variety.
Symptoms such as weight loss, night sweats, anorexia, anemia, hepatosplenomegaly and an elevated LDH can be present in both entities [8]. TB and lymphoma may also share radiologic features. In CT scan, there may be findings of polypoid masses or ulcerations, most frequently near the ileocecal valve; circumferential infiltration; a cavitary mass excavating into the mesentery; endoexoenteric tumors; mucosal nodularity; and fold thickening [9].
The diagnosis of active extrapulmonary TB requires the presence of at least one culture-positive specimen from an extrapulmonary site, histological evidence of AFB, or strong clinical evidence [10]. Confirmatory diagnosis may be difficult to establish given its paucibacillary nature and the invasiveness entailed to access the involved organs, as in this case the liver and the spleen. PCR is done for rapid diagnostic work-up for difficult cases. In India, usually a trial of anti-tuberculosis therapy is started and response is monitored. Good response to treatment suggests correct diagnosis, however in cases of a malignancy masquerading TB poor outcomes are observed and thus contributing to delay in diagnosis [11]. For GI lymphoma, multiple sample sites should be sampled for biopsies for greater diagnostic power [12].
In a study by Patel et al. [10], it was found that hepatomegaly and splenomegaly were present in 18.8% and 12.9% of patients, respectively, but no significant correlation was evident when comparing liver and splenic size with TB. However, the correlation between the presence of splenic micro-abscesses and active TB (diagnosed by smear or culture) was significant (OR 1.9, 95%CI 1.0-3.5, p=0.024) and is related with increased mortality. Thirty eight percent of the time ascites was positively correlated with active TB (OR 2.2, 95%CI 1.2-4.2, p=0.005) [10].
Splenomegaly alone is seen in only two percent of all lymphomas [13], while prevalence data for sole hepatomegaly is unknown. Involvement of both the liver and spleen is only less than one percent for T cell lymphoma.
A retrospective case series showed that the most common GI site involved in NHL is the stomach in up to 68% of cases, followed by the small intestine (20-30%) and the colon and rectum (10-20%) [7,14]. In GI TB, the ileocecal region is the most common site [8]. M. tuberculosis has affinity to the ileocecum due to the relative stasis and abundant lymphoid tissue in this region. The organism penetrates the mucosa and localises in the submucosal lymphoid tissue, where it initiates an inflammatory reaction with subsequent lymphangitis, endarteritis, granuloma formation, caseation necrosis, mucosal ulceration, and scarring [15].
Gastrointestinal perforation may be a complication of both TB and lymphoma; however, the sites of perforation are nonspecific determinants of etiology. While perforation from TB more commonly occurs in the ileocecal region [8,16], that of NHL may also present in the same area. Tuberculous intestinal perforation ranges from 1-15%, which can be attributed to the reactive fibrosis of the peritoneum [17]. For NHL, the predilection for the ileocecal region is unknown; however, it is postulated that the proliferation of lymphoid tissue occurs in the Peyer’s patches in this area [7].
When faced with a dilemma between lymphoma and TB, one has to reach a decision point whether to empirically treat TB first while trying to prove the presence of either or both entities. The response to anti-TB treatment is expected at around two weeks from the start of treatment; however, there is no assurance that intestinal perforation will not occur after starting therapy. Marked improvement while on anti-TB therapy may indicate a correct diagnosis. As in the case written by Puvaneswaran in South Africa, practitioners start empiric antituberculosis treatment with close monitoring for at least one month on all AFB culture-negative patients presenting with such diagnostic dilemma; failure to improve to treatment after the said period must undergo lymph node biopsy [18].
Covering for a curable disease has a greater benefit than expectantly waiting for a histopathological confirmation in a resource-limited setting. Delays in diagnosis may lead to an increase in mortality in such a patient with malnutrition, anemia, hypoalbuminemia, and an overall immune compromised state [5,19]. The presence of acute complications may increase the baseline mortality rate of 4-12% to 12-25% [19].
Tuberculosis is a great mimicker. It is also a disease that can be easily controlled and treated. A very keen clinical eye for the disease is warranted given its high prevalence in the country. The decision to start empiric treatment with anti-TB medications has a higher benefit versus risk of complications, especially in a patient with a high index of suspicion for the disease and where TB burden is high. In this case, it is proven that the ileocecal perforation is due to TB, however the presence of a lymphoma still cannot be totally ruled out.
Download Provisional pdf here
All Sci Forschen Journals are Open Access