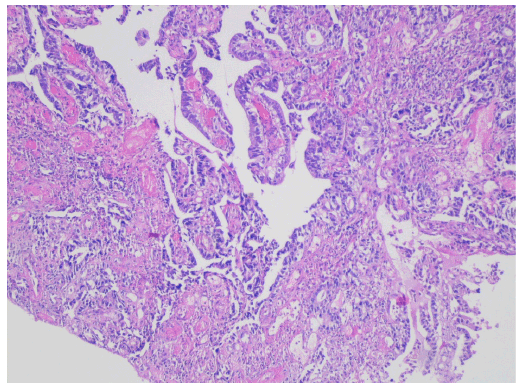
Figure 1: Histopathological examination showing moderately differentiated invasive adenocarcinoma.

Iyikesici MS1 Slocum A2 Turkmen E3 Akdemir O4,* Slocum AK5 Berkarda FB6
1Departments of Internal Medicine and Medical Oncology, School of Medicine, Kemerburgaz University, Istanbul, Turkey*Corresponding author: Akdemir O, Department of Plastic and Reconstructive Surgery, School of Medicine, Kemerburgaz University, Istanbul, Turkey, Tel: +90 532 204 3139; Fax: +90 212 484 1177; E-mail: ovuncakdemir@gmail.com
Aritcle Type: Case Report
Citation: Iyikesici MS, Slocum A, Turkmen E, Akdemir O, Slocum AK, et al. (2016) Complete Response of Locally Advanced (stage III) Rectal Cancer to Metabolically Supported Chemoradiotherapy with Hyperthermia. Int J Cancer Res Mol Mech 2(1): doi http://dx.doi.org/10.16966/2381-3318.120
Copyright: © 2016 Iyikesici MS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
Background: Locally advanced rectal cancer is defined as a rectal mass that cannot be resected without a high probability of leaving residual disease at the tumor site. While the standard treatment for locally advanced rectal cancer is chemoradiotherapy followed by surgery, this study reports a locally invasive rectal adenocarcinoma patient who achieved complete pathological and clinical remission after receiving a combination of metabolically supported chemotherapy (MSCT), radiotherapy (RT) and hyperthermia (HT).
Case presentation: An 81-year-old female underwent a rectosigmoidoscopy at a referring hospital following a complaint of bloody stools for a period of 20 days. The rectosigmoidoscopy revealed an ulcerated tumor beginning at the level of the anal sphincter. A pathological examination of biopsy material revealed moderately differentiated invasive adenocarcinoma and the patient received a diagnosis of stage III (T3N2Mx) lowlying rectal cancer. When further follow-up revealed colonic obstruction, the patient was recommended an abdominoperineal resection (APR) and was referred after refusing surgical treatment. The patient received a metabolically supported combination of oxaliplatin, 5-florouracil (5-FU) and calcium folinate (FOLFOX6) concomitant to RT and local HT and, ultimately, never underwent surgery. 27 months since her disease-free PET-CT scan, the patient remains with no sign of disease recurrence.
Conclusion: According to the findings of the present study, the non-surgical treatment and achievement of complete clinical and pathological remission of locally advanced rectal adenocarcinoma may be possible by means of a combination of MSCT, RT and HT.
Pathological complete response; Locally advanced rectal cancer; Metabolically supported chemotherapy; Hyperthermia
With nearly 1.4 million new cases diagnosed in 2012 and 607,000 deaths recorded in 2008, statistics show that worldwide colorectal cancer is the third most commonly diagnosed cancer among males and the second most commonly diagnosed cancer among females [1,2]. The management of colorectal cancer depends largely on the location of the tumor mass. Nonetheless, surgical resection is the cornerstone of curative treatment [3]. For lower rectal tumors, which are located within up to 6 cm of the anal verge, abdominoperineal resection (APR), with an iliac colostomy, has long been considered the gold standard [4]. If such tumors are also locally advanced (stages II and III), surgical treatment is preceded by preoperative 5-florouracil based concurrent CRT [5,6]. While the standard treatment for locally advanced rectal adenocarcinoma is neoadjuvant chemoradiotherapy (CRT) followed by surgery [6], its treatment by way of metabolically supported chemotherapy (MSCT), radiotherapy and hyperthermia has never been reported.
MSCT is an approach to chemotherapy administration based on Warburg’s hypothesis that “cancer is a disease of metabolic dysregulation” [7] and his 1924 finding that cancer cells carry out glycolysis instead of oxidative phosphorylation for energy generation [8]. In practice, it involves an 8-10 hour fast starting the previous day, the application of pharmaceutical doses of regular insulin and the development of mild hypoglycemia prior to the administration of chemotherapeutic drugs. It is used to enhance the cytotoxic effects of chemotherapeutics and to decrease their toxicity. Hyperthermia (HT), similarly, is a form of treatment that exposes body tissue to temperatures exceeding 42°C or the purpose of improving the tumoricidal effects of both radio and chemotherapy.
Here, the case of a patient with locally advanced rectal adenocarcinoma who achieved complete pathological and clinical remission after receiving a combination MSCT, RT and HT is reported.
An 81-year-old female underwent a rectosigmoidoscopy at a referring hospital following a complaint of bloody stools, severe constipation and abdominal swelling for a period of 20 days. The rectosigmoidoscopy revealed an ulcerated and vegetative tumor beginning at the level of the internal anal sphincter, infiltrating a 7-8 cm segment of the rectum wall and obstructing two-thirds of the distal rectal lumen. A pathological examination of biopsy material revealed moderately differentiated invasive adenocarcinoma (Figure 1). An additional abdominal MRI (magnetic resonance imaging) similarly revealed a circular, diffuse thickening of a 7 cm segment of the rectal wall and narrowing of the intestinal lumen. It did not reveal significant extension to the perirectal area. A CT (computed tomography) performed at the same date with oral and intravenous contrast enhancement of the abdomen and pelvis also revealed several lymphadenopathies, the largest measuring 1 cm, in the perirectal area. A whole body (18F)-florodeoxyglucose (FDG)-PET-CT performed shortly afterwards confirmed the presence of a mass spanning a 5.5 cm segment of the rectum and causing a thickening of up to 22 mm of the rectum wall (Figure 2). While the PET-CT demonstrated intense pathological FDG uptake consistent with primary rectal cancer, the maximum standardized uptake value (SUVmax) being very high (27.2), it did not demonstrate distant metastasis. The patient was diagnosed with stage III (T3N2Mx) distal rectal adenocarcinoma. Even though preoperative 5-florouracil based concurrent chemoradiation (CRT) followed by surgery is the standard of care for locally advanced (stages II and III) rectal cancer [5], because the patient’s tumor was low-lying and the patient developed colonic obstruction upon further follow-up, she was ultimately recommended an abdominoperineal resection (APR) which necessitates a permanent iliac colostomy [9]. Due to the risk of complications and impact on quality of life, the patient reported in the present study was not willing to undergo an APR.

Figure 1: Histopathological examination showing moderately differentiated invasive adenocarcinoma.
The patient was subsequently referred to our hospital for treatment and further management. Blood examination performed upon the patient’s arrival revealed mildly elevated serum carcinoembryogenic antigen (CEA) levels (7.8 ng/mL) and normal carbohydrate antigen 19-9 (CA 19- 9) levels (10 U/ml).
A MSCT protocol consisting of a combination of oxaliplatin, 5-florouracil (5-FU) and ca-folinate (FOLFOX6) to be administered following the application of pharmaceutical doses of insulin was designed for the patient. With the patient’s written, informed consent, this protocol was delivered every week for 3 months. Insulin delivery and chemotherapy infusions were always done in conjunction with blood glucose monitoring. Concomitant to MSCT; the patient also received external beam radiation therapy, which was administered at a total dose of 50.6 Gy in 22 fractions given 5 days a week over a period of one month. The patient received local HT once weekly throughout the duration of MSCT, subsequent to each chemotherapy session. The Oncotherm (Troisdorf, Germany) EHY-3010 hyperthermia device, which uses electromagnetic energy and produces a modulated electric field, with a carrier frequency of 13.56 MHz, that is generated by two active electrodes and that passes through the patient’s body, was employed in the stimulation of heat. Throughout the hyperthermia sessions, while a mobile electrode measuring 30x40 cm was positioned at the pelvic colorectal area, a second stationary counter electrode remained in a fixed position below the patient. In accordance with the consensus guidelines determined by the American Society of Peritoneal Surface Malignancies, each hyperthermia session lasted 60 minutes [10]. Also in accordance to the consensus guidelines, heating up to 44 C was achieved by gradually increasing the power of the electrodes from 80 W (Watts) to 130 W [8]. Following 12 sessions of MSCT and HT, as well as 22 sessions of RT, the patient underwent a second PET-CT (Figure 3). Evaluation of the PET-CT demonstrated disease response, with the primary tumor in the rectum displaying recession. While the tumor originally spanned a 5.5 cm segment of the rectum, and had a SUVmax of 27.2, the second PET-CT report revealed an involvement of a 3 cm segment of the rectum wall and a SUVmax of 3.9, which was evaluated as being related to radiation induced inflammation. The patient continued receiving the same metabolically supported chemotherapy protocol followed by 60-minute local hyperthermia sessions for a 6-month-period after the second PET-CT evaluation. A PET-CT evaluation at the end of 6 months revealed no FDG uptake consistent with malignancy. The patient was referred for a low anterior resection, which the patient once again refused. 27 months after the patient finally disease-free PET-CT and remains free of pathology. Routine follow-up examinations, with CEA and CA 19-9 screenings and an abdominal CT evaluation every 3 months, have revealed no evidence of recurrence.
Locally advanced rectal cancer cannot be resected without a high probability of leaving microscopic or gross residual disease at the original tumor site due to tumor fixation or adherence to neighboring tissue [9]. Hence, standard treatment in the case of most patients with locally advanced disease incorporates multiple modalities, including preoperative CRT, surgery and postoperative chemotherapy [11]. The significance of preoperative CRT has been widely researched and that preoperative CRT achieves high rates of local control and five-year overall survival rates of about 60 percent for patients with locally advanced rectal cancer has been reported in several retrospective studies [12-15]. Standard CRT consists of 5-FU-based infusionalchemotherapy and concurrent fractionation RT (45 to 50 Gy administered over a period of 5.5 to 6 weeks) [16]. Surgical exploration is undertaken four to eight weeks after neoadjuvant CRT and additional chemotherapy is administered following surgery [9]. Of the treatment modalities employed in the treatment of the present case, metabolically supported chemotherapy is based on the Warburg hypothesis, a phenomenon also referred to as the “Warburg effect”. According to this hypothesis, deficiency in oxidative phosphorylation and elevated glycolysis in cancer cells are the primary causes of cancer and a dysregulation of the metabolism at a cellular level is the cause for progressive transition from normalcy to malignancy [17]. MSCT is centered on various types of metabolic interventions directed towards correcting this metabolic dysregulation at a cellular level. In practice, the metabolic modification of cancer cells is achieved through the use of insulin, which together with insulin like growth factors I and II (IGFs), is central to the mechanism of autonomous growth and malignancy in cancer cells [18]. Studies suggest that insulin enhances the cytotoxic effects of chemotherapeutic drugs, first of all, by increasing membrane fluidity and permeability, therefore, allowing for increased drug penetration [16,19-21]. The adsorption of drug molecules onto insulin and the formation of drug-insulin complexes, later internalized by receptormediated endocytosis, have also been discussed as causes for increased intracellular drug levels and resulting increased cytotoxicity [22-25]. The metabolic modification that insulin brings about in cancer cells is, most importantly, related to the cross reaction of insulin with IGF receptors located on cancer cell membranes. This cross-reaction extends the S-phase fraction of the cell cycle [26] and ultimately renders cancer cells more susceptible to the cytotoxic effects of anticancer drugs, especially in the case of cell-cycle phase-specific agents [27]. Therefore, adding insulin to chemotherapy regimens allows for the use of lower systemic drug doses with increased efficacy – as higher intracellular doses are achieved within cancer cells –and an increased level of safety – as the lower concentration of insulin receptors on normal cells spares them from the intensity of the cytotoxic effects of chemotherapeutic drugs [28].
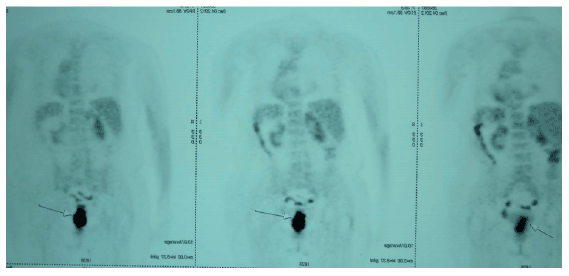
Figure 2: Whole body (18F)-florodeoxyglucose (FDG)-PET-CT showing the presence of a mass, spanning a 5.5 cm segment of the rectum associated with rectal wall thickening.
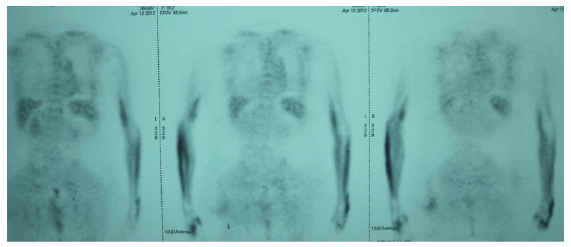
Figure 3: Follow-up PET-CT showing shrinkage of the primary tumor and decrease in rectal wall thickening.
HT is the second form of non-standard treatment the present case received. HT is defined as a form of treatment in which body tissue is exposed to high temperatures (up to 45°C) to damage and kill cancer cells or to make cancer cells more sensitive to the effects of radiation and certain anticancer drugs [29,30]. The antitumor effects of HT have been clearly demonstrated [31-34]. Numerous clinical trials carried out since the early 1970’s, have shown that HT significantly improves the effectiveness of both CT and RT by interfering with the repair of radiation- induced DNA damage [35] and through synergistic interaction with cytotoxic drugs [36]. When RT is administered in combination with HT, in vivo studies have demonstrated that the effect of radiotherapy can be enhanced by a factor of between 1,2 and 5 [37,38]. HT is also reported to be the most potent radiosensitizer to date. That the administration of chemotherapy agents, including platin analogues, alkylating agents and nitrosureas, together with HT, enhances the efficacy of these drugs by a factor of between 1,2 and 10 in virtually all cell lines has also been reported [39]. General discomfort, patient anxiety and pain due to the “hot-spot phenomenon” (specific acute to subacute side effects caused by electrical interface) have been reported as common problems related to the local HT of deep-seated tumors in up to 60% of patients [40]. The patient reported in the present study experienced mild discomfort during the first 3 sessions of HT. This discomfort later resolved. The patient experienced no such late toxicity symptoms as chronic bowel dysfunction, ulceration of the bowel or bladder or obstruction/stricture of the ureters. Such signs of toxicity were not observed in any patients included in a phase II clinical trial carried out by Rau B et al. either [41].
Although there are no reports of the non-surgical treatment of locally advanced low-lying rectal adenocarcinoma using RT and HT combined with MSCT, various non-randomized and randomized trials for rectal cancer have demonstrated an improved local response with the combined use of HT and standard radiochemotherapy [42]. One randomized trial that employed endocavitary HT in addition to radiotherapy with locally advanced T4 tumors reported that radical resection rate was significantly increased in the combined treatment group (55% vs. 27.1% with radiotherapy alone) and that the patients in the combined treatment group also had significantly improved 5-year survival rates (36% vs. 7%) [43]. Using similar techniques as the previously mentioned trial, Mori et al. treated 11 patients with deep-seated rectal cancer (Dukes’ A-C) and reported benefit in 6 of 11 cases [44]. A phase III trial that treated 146 patients with rectal cancer (Dukes’ A-C) either by preoperative RT and HT or preoperative RT alone, with patients in the control arm appointed for surgery with no preoperative treatment, reported significantly increased complete response rates (22.7% vs. 5.3%) and 5-year survival rates of 66.7% versus 50% after RT alone and 40.5% in the control arm [45]. In contrast to the patients in the previously mentioned studies, however, the case reported at present received MSCT and never underwent a resection. Regardless of never having undergone surgery, the patient has maintained a disease-free status.
To our knowledge, this is the first study discussing the use of MSCT, RT and HT for the treatment of locally invasive rectal adenocarcinoıma. Considering the benefit that the present case received, further research is necessary in these areas for the standardization of such treatment modalities.
This study, which is limited to one patient and discusses a 27-month follow-up period, demonstrates that the non-surgical treatment and achievement of complete clinical and pathological response is may be possible by means of a combination of MSCT, RT and HT.
Download Provisional pdf here
All Sci Forschen Journals are Open Access