
Table 1: Predictors of overall survival (Univariate analysis)
Abbreviation: *
violated proportionality hazards assumption and was not used in analysis; ECOG, Eastern Cooperative Oncology Group.

Koul R1* Narasimhan G2 Dubey A1 Tai P3
1Cancer Care Manitoba, McDermot Ave, Winnipeg, MB, Canada*Corresponding author: Koul R MD, FRCPC, Department of Radiation Oncology, 675 McDermot Ave, Winnipeg, MB, Canada R3E0V9, Tel: 204-7871400; Fax: 204-7860194; E-mail: rkoul@cancercare.mb.ca
Aritcle Type: Research Article
Citation: Koul R, Narasimhan G, Dubey A, Tai P (2015) A Clinical Review and Treatment Outcome in Glioblastoma: Is Age Only a Number? Int J Cancer Res Mol Mech 1(4): doi http://dx.doi.org/10.16966/2381- 3318.118
Copyright: © 2015 Koul R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
Introduction: Overall incidence rate of all brain tumors to be 10.82 (95% CI: 8.63–13.56) per 100,000 person-years. Glioblastoma accounts for up to 60% of all malignant primary brain tumors in adults, occurring in 2-3 cases per 100,000 in Europe and North America. In 2005 maximum safe surgical resection, followed by radiotherapy with concomitant temozolomide (TMZ), followed by adjuvant TMZ became the standard of care for glioblastoma. We adopted this as the standard of care in 2009 in the province of Saskatchewan, Canada.
Material and methods: A cohort of 393 consecutive patients with pathologically proven glioblastoma, who had been registered in the Province of Saskatchewan from 2000 to 2010, was examined. Survival analysis was performed using Kaplan-Meier curves and log-rank test for comparing subgroups. The independent effect of factors that predicted survival at the bivariate level was determined using a Cox proportional hazard model.
Results: Median age at diagnosis was 67 years in females and 63 years in males. The median overall survival was 13.8 months (95% CI: 12.6, 15.1 months). Based on a literature review and after the univariate analysis, the following variables were included in the Cox’s multivariable model: age at diagnosis, ECOG status (dichotomous variable created), type of surgery (complete vs. sub-total), and whether chemotherapy and radiotherapy were given after surgery. Patients who treated with chemotherapy and chemotherapy had a better median survival of 18.1 months vs. 11.3 months without chemotherapy. Patients younger than 50 years did better as compared to elderly population. For fit elderly patients >70 years, 11.0 months median survival was achieved. Contrary to common belief in literature, patients with headache do not have a worse survival and patients who presented with seizure survived better.
Conclusion: Our series demonstrates improved survival outcomes for patients <50 years old quite consistent with literature. However for elderly patients with excellent performance status in the western Canadian province, a median survival of <70 years 16.1 months (95% CI 13.6- 18.1 months) ≥ 70 years 11.7 months (95% CI: 10.4-13.0 months) was achieved which is better than other series in the current literature. The landscape of treatment options for GBM patients has changed substantially over the past decade and with further information still amassing in ongoing clinical trials in GBM population the suggestion is to base treatment options based on patient age and KPS. Until further treatment advances are made for GBM in general, utilizing the current therapeutic options of surgery, RT, and TMZ appropriately according to patient age, performance status, and patient preferences represents optimal management.
Glioblastoma, a WHO grade IV tumor, is the most aggressive primary brain tumors, accounting for 17 percent of all CNS malignancies [1]. In patients over the age of 60, the rate of glioblastoma greatly increases, and thus accounts for the majority of primary brain tumors in this population. Despite recent advances in treatment, the prognosis for patients with glioblastoma is dismal. The overall survival rates after diagnosis have been reported to range between 5 and 12 months; long-term survivors are usually young, with good performance status and able to undergo multimodality treatment for their disease [2]. In 2005 maximum safe surgical resection, followed by radiotherapy with concomitant temozolomide (TMZ), followed by adjuvant TMZ became the standard of care for glioblastoma. We adopted this as the standard of care in 2009 in the province of Saskatchewan.
A retrospective consecutive cohort of 393 patients with pathologically proven glioblastoma, who had been registered in the Province of Saskatchewan from 2000 to 2010, was examined. Survival analysis was performed using Kaplan-Meier curves and log-rank test for comparing subgroups. The independent effect of factors that predicted survival at the bivariate level was determined using a Cox proportional hazard model.
Since GBM is the cause of death for the large majority of the patients in this cohort (96%), one would not reasonably expect cause-specific survival to vary significantly compared to overall survival. Death information is only missing for 12 patients in the cohort. Therefore overall survival has been used as the outcome measure in the analysis.
The basic demographics and univariate analysis are illustrated in table 1. Median age at diagnosis was 67 years in females and males was 63 years. Median overall survival in our retrospective study was 13.8 months (95% CI: 12.6, 15.1 months, Figure 1). Figure 2 shows the difference in survival with and without chemoradiation. Based on a literature review and after the univariate analysis, the following variables were included in the Cox’s multivariable model: age at diagnosis, Eastern Cooperative Oncology Group (ECOG) performance status (dichotomous variable created), type of surgery (complete vs. sub-total), and whether chemotherapy and radiotherapy were given after surgery (Table 2). Figures 3a and 3b show the overall survival of different age groups using different cutoffs. Patients with headache (Figure 4) and patients who presented with seizure survived better (Figure 5).

Table 1: Predictors of overall survival (Univariate analysis)
Abbreviation: *
violated proportionality hazards assumption and was not used in analysis; ECOG, Eastern Cooperative Oncology Group.
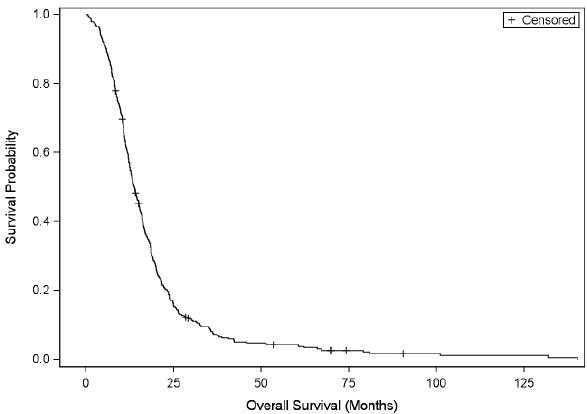
Figure 1: Kaplan-Meier estimates of overall survival in evaluable patients (N=393)

Figure 2: The difference in survival with and without chemoradiation.
Our study consisted of unselected consecutive patients, unlike in a clinical trial setting. From the literature, surgery is the initial recommended approach for both debulking and to obtain histological diagnosis [3]. In our study, we have added GTR and near total resection together so cannot discuss one approach is better than other. There was considerable debate in the literature regarding the impact of the extent of resection on overall survival in patients with glioblastoma. While some studies have failed to show a benefit with more complete tumor resection, others have demonstrated an increase in overall survival for patients with glioblastoma who undergo more complete resections of their tumors [4]. Whenever possible, safe, maximal resection is preferred in the management of glioblastoma. Further resection after initial biopsy is left to the discretion of the neurosurgeon depending on the location of tumor [5]. Several recent systematic reviews have addressed the issue of survival benefit for gross total resection versus partial resection in patients with glioblastoma [6,7]. In a thorough systematic review of the literature up to 2004, Taylor et al., along with the Neuro-oncology Disease Site Group of Cancer Care Ontario reviewed five retrospective studies and five prospective studies comparing gross total resection (GTR) to subtotal resection (STR) in terms of survival [8]. Apart from one preliminary prospective analysis published in 1990, all of the studies included in their review reported a significant improvement in survival for patients undergoing GTR compared to STR (p<0.05). However, the authors identified several confounding factors, including the trend for more aggressive surgery in younger patients with a better KPS score, and therefore recommended that the results be interpreted with caution [9].
Adjuvant chemo-radiation therapy is considered the standard of care following surgery for patients with newly diagnosed glioblastoma, although the role in the very elderly needs more research [10]. Whenever possible, surgery should be followed by radiotherapy and concurrent temozolomide chemotherapy, followed by six cycles of adjuvant temozolomide as per Dr Stupp’s trial [11]. For patients who show improvement on therapy, additional cycles of temozolomide may be considered [12]. External beam radiation therapy should be given in standard fractionation to a maximum total dose of 60 Gray (Gy, the radiation absorbed dose) using 3D-conformal planning techniques or IMRT (intensity modulated radiotherapy) technique if available in institutions. There is no strong evidence to recommend a total dose greater than 60 Gy in standard fractionation, and alternative fractionation schedules have not proven to be more beneficial [13]. However the Nordic trial showed for patients older than 70 years, survival was better with hypofractionated radiotherapy of 34 Gy/10 fractions than with standard radiotherapy of 60 Gy/30 fractions (HR for 0.59 [95% CI 0.37-0.93], p=0.02). In our series, <70 years 16.1 months median survival was achieved (95% CI 13.6-18.1 months) and ≥ 70 years , 11.7 months (95% CI: 10.4-13.0 months). The results in our series were better than the Toronto series in which the median survival rates for <70 years and ≥ 70 years were about 8 and 5 months respectively [14].

Table 2:Prognostic factors influencing overall survival -Multivariable
analysis, Final model
Each variable in multivariable analysis is compared to a referent category.
A significant effect would be that the hazard of experiencing the event for
patients in a group is different from the hazard of experiencing the event in
referent category. For example, a hazard ratio of 1.03 means that patients
over 50 years of age have a 3% higher hazard than patients who are 50
years or younger.
Management of the elderly patients is still controversial. While retrospective studies suggest combined hypofractionated radiotherapy and temozolomide were well tolerated and treatment results are better than monotherapy, but prospective results are not available yet [15]. A retrospective series from Connecticut showed similar overall survival between hypofractionated and standard fractionation in the setting of temozolomide for older glioblastoma patients [16]. The ongoing European Organization for Research and Treatment of Cancer/National Cancer Institute of Canada trial will help clarify the role for concurrent TMZ with hypofractionated RT fractionation [17].
However for elderly patients with poor KPS, reasonable options include best supportive care, TMZ alone, hypofractionated RT alone, or whole brain RT for symptomatic patients needing to start radiation treatment urgently. Given the balance between short survival and quality of life in this patient population, optimal management of elderly GBM patients must be made individually according to patient age, MGMT methylation status, performance score, and patient preferences [18].
Another important prognostic factor is the grade and proliferative index of tumor. The higher the grade, the more malignant the tumor is and the worse prognosis [19]. Tumors are graded mainly on the basis of their proliferation index, which is an important prognostic factor in glioblastoma. The Ki-67 protein is expressed in all phases of the cell cycle except G0 and serves as a good marker for proliferation. Studies that have evaluated proliferation index by Ki-67 immunohistochemistry in GB have shown a significant correlation between high proliferation rates and shorter disease-free and overall survival [20]. Cytogenetic and molecular genetic studies of GB have shown that the most frequent alterations encountered in these tumors are loss of heterozygosity on chromosome arm 10q (60%–90%), mutations in p53 (25%–40%), PTEN mutations (30%), overexpression of MDM2 (10%–15%) and epidermal growth factor receptor (EGFR) gene amplification [21]. In one study it was found that being younger than age 40 years is strongly associated with a favorable prognosis. EGFR amplification, loss of 9p21 and gain of chromosome 9 had prognostic significance for all patients, whereas gain of chromosome 7 and loss of 10q23/PTEN showed clinical importance only for patients age 40 years and older [22]. Interestingly, the protein product of MGMT gene, 06 alkylguanine–DNA alkyltransferase, was shown to be involved in tumor resistance to alkylating agents [23]. Silencing of the MGMT gene by promoter methylation compromises DNA repair and has been associated with longer survival in patients with glioblastoma who receive alkylating agents [24]. High levels of MGMT activity in cancer cells create a resistant phenotype by blunting the therapeutic effect of alkylating agents and may be an important determinant of treatment failure [25]. Silencing of the MGMT gene by promoter methylation is associated with loss of MGMT expression 11-13 and results in lower DNA-repair activity thus decrease in tumor formation. So, gene silencing by DNA methylation will be the very important mechanism by which tumor-suppressor genes will be inactivated thus decreasing chances of treatment failure and lower outcomes [26].

Figure 3: Figures 3a and b show the overall survival of different age groups using different cutoffs.
(a): Age groups specified are as follows: <=60, 61-70, 71-80, 80+ (b): Age groups specified as follows: <=69, 70-75, 75-80, 80+

Figure 4: Headache as presenting symptom

Figure 5: Seizure as presenting symptom
Limitations of our study include the retrospective data and lack of modern molecular biomarkers in most patients: O6-methylguaninemethyltransferase (MGMT) and isocitrate dehydrogenase 1 (IDH1) status were not available in earlier years [25]. Determination of MGMT promoter methylation status may assist in determination of prognosis.
In conclusion, the extent of resection, age of the patient, performance status, location and volume of the tumor have been identified as important prognostic indicators of overall survival in patients with glioblastoma. In our series elderly patients also did well as compared to other data in literature .This benefit may be due to good performance status as most of them are farmers and lead an active life.
Download Provisional pdf here
All Sci Forschen Journals are Open Access