Article Information
Aritcle Type: Review Article
Citation: Jiang Liu L, Huang J (2015) Regulation of
Epithelial-Mesenchymal Transition by Transcriptional
Factors in Cervical Carcinoma. Int J Cancer Res
Mol Mech 1(3): doi http://dx.doi.org/10.16966/2381-
3318.111
Copyright: © 2015 Jiang Liu L, et al. This is an
open-access article distributed under the terms
of the Creative Commons Attribution License,
which permits unrestricted use, distribution, and
reproduction in any medium, provided the original
author and source are credited.
Publication history:
Received date: 15 Jun 2015
Accepted date: 12
Sep 2015
Published date: 17 Sep 2015
Abstract
Cervical carcinoma is a most prevalent cancer in women worldwide. The metastasis is one of the major issues for late-stage cervical carcinoma
in patients. Epithelial-mesenchymal transition (EMT) has been implicated in cervical carcinoma progression and metastasis. During EMT, cervical
carcinoma cells lose epithelial features and gain a mesenchymal phenotype. The EMT has been identified to be regulated by key transcription
factors including Snail, Zeb, and Twist. In this review, we will discuss our current understanding of how these key transcription factors play
important roles in EMT program of cervical carcinoma cells.
Keywords
Transcription factors; Epithelial-mesenchymal transition; Cervical carcinoma
Cervical Carcinoma
Cervical carcinoma is a most prevalent cancer and a common cause
of death in women worldwide. The major types of cervical carcinoma
include squamous cell carcinoma (SCC) and adenocarcinoma [1].
Squamous cell carcinoma begins in the thin and flat cells that line the
cervix while adenocarcinoma begins in cervical cells that make mucus
and other fluids [1]. About 90% of cervical carcinoma is squamous cell
carcinoma, only 10% of cervical carcinoma is adenocarcinoma [1]. The
major etiological factor of cervical carcinoma is the presence of human
papillomavirus (HPV) oncogene [2]. HPV can be classified into highrisk
and low-risk types. High-risk HPVs such as HPV-16, 18, and 31
are associated with more than 90% cervical carcinoma [2]. HPV is
contributing to progression of cervical carcinoma through the action
of HPV oncoproteins E6 and E7 which interact with tumor suppressor
proteins such as p53 and pRB to interfere critical cell cycle [3]. E6 and E7
are invariably expressed in HPV-positive cervical carcinoma cells and play
important roles in carcinogenesis and maintenance of the transformed
phenotype [3]. Despite HPV are thought to be the major cause of cervical
carcinoma, however, our data and others have shown that HPV alone is
not sufficient to induce cervical carcinoma formation, suggesting that the
factors other than HPV viral proteins also contribute to the progression
of cervical carcinoma [4-6].
The metastasis is one of the major issues in late-stage cervical
carcinoma in patients. In order to migrate, cancer cells need to activate
genes for cellular proliferation, change cellular characteristics from
epithelial to mesenchymal, activate anti-apoptotic signaling to initiate
cell differentiation, down-regulate the receptors to help cell-cell
attachment, up-regulate the cell adhesion molecules to promote cell
movement, degrade cell-cell junctions, and activate proteases at the
cell surface [7]. Whether or not a cancer cell successfully migrates
for metastasis is related with cancer progenitor cell characteristics,
environmental factors, extracellular and intracellular signaling, and
epigenetic changes all influence [8].
Epithelial-mesenchymal Transition in Cervical Carcinoma
Epithelial cells have distinctive features of cell adhesion and apical-basal
polarity, whereas mesenchymal cells loose cell adhesion and have a frontback
cell polarity [9]. Epithelial cells can be converted to mesenchymal
cells through a epithelial-mesenchymal transition (EMT) process in many
cancers including cervical carcinoma, which have dramatic phenotypic
changes by the loss of epithelial marker proteins such as E-cadherin and
the acquisition of mesenchymal marker proteins such as vimentin [9-
12]. It has been proposed that three types of EMT are involved in cancer
progression. The type 1 of EMT is in the developmental processes, type
2 of EMT is in the inflammation, tissue remodeling, wound healing, and
fibrosis, and type 3 of EMT is in cancer invasion and metastasis [13].
The process of EMT is reversible when mesenchymal cells gain epithelial
characteristics via mesenchymal-epithelial transition (MET) process [14].
Interestingly, incomplete EMT in an epithelial cancer cell may generate a
combine metastable cell which contains both epithelial and mesenchymal
phenotypes and consistent with the existence of cancer cells in various
tumors including cervical carcinoma [1].
The epithelial-mesenchymal transition plays an important role in
metastasis of cervical carcinoma. The transfection of oncoproteins E6 and
E7 in cervical carcinoma cells showed the up-regulation of mesenchymal
markers SMA and vimentin and the down-regulation of epithelial
marker E-cadherin during EMT [15]. Loss of E-cadherin is related to the
oncoprotein E5 of human HPV, while forced expression of E-cadherin in
the immortalized cell line with oncoproteins E6 and E7 can reverse
the invasive phenotype [16]. The promoter DNA hypermethylation
is a major contributor that regulating transcription activity of the
E-cadherin gene and the hypermethylated DNA is detectable in serum
of cervical carcinoma patients [17]. E-cadherin expression can be
reactivated using HDAC inhibitor valproic acid (VPA), suggesting
that histone modification and chromatin remodeling is involved in
the regulation of E-cadherin expression in cervical carcinomas [17].
Although hypoxic has been suggested to be involved in E-cadherin
suppression in cancers, however there is no evidence to show that
the oxygenation is directly related with E-cadherin expression in the
squamous cell carcinoma of uterine cervix [18].
Loss of E-cadherin during EMT
E-cadherin is expressed primarily in epithelial cells as a single-span
transmembrane glycoprotein of five repeats and one cytoplasmic domain
[19]. E-cadherin mediates cell-cell adhesion via interacting with a number
of proteins including α-catenin, β-catenin, and p120 catenin which link
E-cadherin to the actin cytoskeleton in its cytoplasmic domain [20]. The
extracellular domain of E-cadherin contains characteristic repeats that
regulate homophilic and heterophilic interactions [21]. The evidences
suggest that the combination of cis-dimerization of two cadherin molecules
on the same cell surface and trans-interactions between cadherin dimers
on opposing cell surfaces which maximizes homophilic adhesion [22-25].
Loss of E-cadherin is a common feature of EMT in epithelial cancers
including cervical carcinoma, which has been found to increase cancer
cell invasion and metastasis [7]. E-cadherin is a tumor suppressor of many
tumors and its down-regulation provokes the development of malignant
epithelial cancers. Several important transcription factors have been
shown to associate with E-cadherin during EMT. As a member of the
Snail family of transcriptional repressors, Slug is capable of repressing
E-cadherin expression to trigger EMT, suggesting that it may play a role
as an invasion promoter. The evidence suggests that both Snail and its
family member Slug are capable of repressing E-cadherin in epithelial
cells via the E-box elements in the proximal E-cadherin promoter [26].
Behrens et al. [27] have demonstrated that epithelial cells assume invasive
characteristics due to loss of E-cadherin-mediated cell adhesion. Burdsal
et al. [28] have shown that blocking E-cadherin is sufficient to trigger EMT
in mammalian cell systems. Therefore, loss of E-cadherin is frequently
associated with strong invasive tendencies and can be considered as a
classical marker of poor prognosis of cervical carcinoma.
Regulation of EMT by Transcription Factors in Cervical Carcinoma
Many transcription factors have been reported to associate with the
regulation of EMT. These transcription factors include the Snail family
of zinc-finger transcription factors such as Snail1 (Snai1), Snail2 (Slug),
and Snail3 (Smuc); the two-handed zinc-finger factors of d-crystallin/E2
box factor family proteins zinc-finger E-box-binding homeobox (Zeb)1
and Smad-interacting protein Zeb2; and the basic helix-loop-helix factors
Twist1 and Twist 2 [17,29,30]. These transcription factors recognize
the DNA sequences of E-box in the promoter region of E-cadherin
and recruit cofactors and histone deacetylases resulted in repressing
E-cadherin expression [31]. In addition, these transcription factors act as
molecular switches response to the signaling pathways and regulate the
EMT program [32].
Snail, Slug and Smuc
The Snail family proteins include Snail (Snai1), Slug (Snai2), and Smuc
(Snai3) which are zinc finger transcriptional regulators [33]. The Snail
family proteins encode transcription factors of the zinc-finger type and
share a highly conserved carboxy-terminal region and a divergent aminoterminal
region [34]. The zinc-finger type includes the cysteines and
histidines (C2H2) type and function as sequence-specific DNA-binding
motifs [35]. The amino-terminal part of the zinc-finger type can bind to a
major groove of the DNA [36]. In addition, the zinc-finger type includes
two beta-strands followed by alpha-helix [36]. The two conserved C2H2
coordinate the zinc ion [37]. It has been shown that the consensus binding
site of Snail-related genes contains a core of six bases, CAGGTG [38]. This
motif is identical to the core binding site of basic helix-loop-helix (bHLH)
transcription factors [39], suggesting that Snail proteins might compete
with them for the same binding sequences.
Snail has been shown to convert normal epithelial cells into
mesenchymal cells through the direct repression of E-cadherin expression
[40]. More importantly, Snail knockout mice die at gastrulation stages
and show defects in EMT [41]. Mutant embryos retain all characteristic
of epithelial cells with apical–basal polarity, microvilli and Adherens
Junctions [42]. This study indicates that Snail acting as a repressor
of E-cadherin expression and loss of Snail proteins in epithelial cells
resulted in failing to undergo EMT. Snail and Slug are considered major
transcription factors that regulate EMT in various cancers including
cervical carcinoma [9,43,44]. Several studies have shown that Snail family
proteins play important role in induction of EMT in cervical carcinoma
[1,6] (Figure 1). The Snail inhibits the expression of claudins, occludin, and
thrombomodulin in cervical carcinoma cells [1,45,46]. Snail and Smuc
have been reported to associate with lymph node metastasis [47]. It was
shown that Snail and Slug bind to the E-cadherin promoter, up-regulate
mesenchymal makers such as Vimentin, and ultimately promote EMT
[48]. The up-regulation and nuclear accumulation of Snail are correlated
with EMT in cervical carcinoma [47]. These data suggest that Snail family
proteins play important role during EMT in cervical carcinoma.
Zeb1 and Zeb2
Zeb family proteins including Zeb1 and Zeb2 are sequence-specific
DNA-binding transcription factors [49]. Several studies have shown
that Zeb1 and Zeb2 can regulate E-cadherin expression in multiple
human cancers through binding the E-boxes of E-cadherin [17,50,51].
Both Zeb1 and Zeb2 contain the helix-loop-helix motif that bind to the
bipartite E-boxes of E-cadherin promoter region [17]. Polycomb protein
Pc2 is required for E-cadherin repression mediated by small ubiquitinlike
modifier (SUMO) conjugated lysine residues Lys391 and Lys866 in
Zeb proteins [17,52]. In addition, Zeb proteins control the microRNA
expression by interfering in microRNA promoter activity to form a
reciprocal feedback loop in EMT [53]. Dysregulation of both Zeb1/2
and E-cadherin can be found in a lot of tumorigenic processes such as
the stem-like cell character, development of mesenchymal phenotype,
aggressiveness in EMT, resistance to therapeutic agents, adaptive stages
under hypoxic microenvironment, and cancer progression [17,54 55].
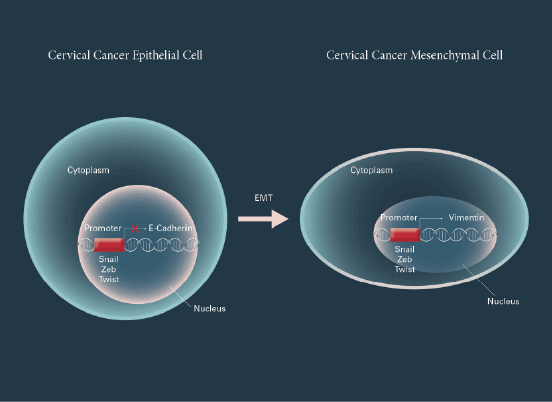
Figure 1: Transcription factors regulate EMT in cervical carcinoma cells
Cervical cancer epithelial cell can be converted to cervical cancer
mesenchymal cell by regulation of Snail, Zeb, and Twist family proteins
in nucleus, respectively during an epithelial-mesenchymal transition
(EMT) process, which has dramatic phenotypic changes by the loss
of epithelial marker proteins such as E-cadherin and the acquisition of
mesenchymal marker proteins such as vimentin.
Normally, mesenchymal cells highly express Zeb1, whereas epithelial
cell lack Zeb1 expression [56]. Zeb1 can induce EMT through suppressing
E-cadherin and other genes to participate in epithelial cell polarity, when
Zeb1 is inappropriately expressed in cervical carcinomas [57]. Nuclear
Zeb1 expression is detected in most of invasive cervical carcinomas
[58]. In addition, Nuclear Zeb1 expression is associated with high
grades in cervical carcinoma [35]. Although hypoxic has been suggested
to be involved in E-cadherin suppression in solid tumors, however the
oxygenation status has no direct correlation with E-cadherin level in the
cervical carcinoma [18]. Clinically, Zeb1 expression has been found in
more than 95% cervical carcinoma and the expression level of Zeb1 was
significantly associated with International Federation of Gynecology and
Obstetrics stages and regional lymph node metastasis [17]. At present,
whether Zeb1 and Zeb2 are involved in the cervical carcinomas remained
to be determined.
Twist1/2
Twist is a transcription factor protein that belongs to the family of basichelix-loop-helix
proteins (bHLH) [20]. Twist includes a conserved domain
with two α-helices separated by an inter-helical loop [59]. Twist can form
dimers by its helices and binds to the DNA sequences 5’-CANNTG-3′
called E-boxes [60]. In vertebrate animals, Twist encodes two similar
genes, Twist1 and Twist2 which are 90% identical. The C-terminal
sequence of E-box in Twist is associated with the anti-osteogenic function.
Twist1 has a glycine-abundant region in the N-terminal of E-box, whereas
Twist2 does not have such region. Both Twist1 and Twist2 are associated
with the differentiation of muscle, cartilage and osteogenic cells [61].
Twist is mainly found in neural crest cells in vertebrates [62]. The absence
of Twist2 function in mice is associated with cachexia [60].
Twist family proteins have been reported to contribute in tumor
metastasis by promoting EMT [63]. Twist2 protein regulates E-cadherin
expression by down-regulating E-cadherin promoter activity [64]. Twist1
is a master regulator and a primary cause of EMT in cervical carcinoma
[22,35]. The expression of Twist1 is associated with chemotherapy
and radiotherapy resistance while the inactivation of Twist1 by RNA
interference induces cell apoptosis in cervical carcinoma cells [65]. In
addition, the overexpression of Twist1 leads to a poor prognosis and
the knockdown of Twist1 induces down-regulation of MDR1/P-gp
(multi-drug resistance protein) expression, inhibiting its efflux activity,
and sensitizing cervical cancer cells to cisplatin treatment in cervical
carcinomas [66]. Twist2 expression in cervical squamous cell carcinoma
patients is a predictor for metastatic potential and Twist2 increases the
rate of migration and invasion more than Twist1 [67]. Twist plays a
role in the regulation of EMT in cervical cells through maintaining the
CD44 expression and stem cell-like properties associated with EMT [68].
The expression of Twist is critical for EMT induction by increasing the
expression of CD44, enhancing tumor sphere formation, and promoting
ALDH1 activity during cervical carcinoma development [8]. Twist
induces the activation of β-catenin pathway and Wnt3 signaling in Twistoverexpressing
cells [68]. The aberrant expression of Twist1 and Twist2
in cervical carcinoma cells is associated with activation of AKT pathway
resulted in phosphorylation and suppression of GSK-3b [40]. These
data suggested that both Twist1 and Twist2 play important role through
regulation of EMT during cervical carcinoma development.
Conclusion
Various transcription factors have been reported to associate with the
regulation of EMT in cancer. In this review, we discussed how some of the
transcriptional factors such as Snail, Zeb, and Twist proteins play important
roles in EMT during cervical carcinoma development. Metastasis is the
major cause of death in cervical carcinoma and EMT plays a key role in
metastasis of cervical carcinoma by down-regulation of epithelial marker
E-cadherin and up-regulation of mesenchymal marker vimentin, resulted
in increasing cancer cell survival, migration, invasion, metastasis, and
recurrence. Interestingly, many studies have shown that activation of EMT
transcriptional factors is associated with oncogenic transformation which
make them more aggressive and promote the development of metastatic
properties. As molecular switches, these activated EMT transcriptional
factors can respond to complex signaling pathways and regulate the EMT
program. In addition, these activated EMT factors can recognize the E-box
DNA sequences in the promoter region of E-cadherin, recruit cofactors
and histone deacetylases to repress its expression. Therefore, these
activated EMT transcriptional factors have been implicated in the cancer
stem cell property, cancer recurrence, resistance of radio therapeutic and
chemotherapeutic drugs, and immune suppression. Studies in cell lines
and xenograft mice models have identified that the function of activated
EMT transcriptional factors in cancer is not only as important diagnostic
and prognostic biomarkers, but also as potential therapeutic targets.
Taken together, a better understanding the role of transcriptional factors
in promoting EMT and cancer stem cells in cervical carcinoma will lead
to develop more new prognostic biomarkers and therapeutic targets for
cervical cancer invasion and metastasis.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the
publication of this paper.
Acknowledgments
This study was supported by a grant from National Natural Science
Foundation of China to LJL.


