Article Information
Aritcle Type: Research Article
Citation: Karbowski LM, Murugan NJ, Dotta BT, Persinger MA (2015) Only 1% Melanoma Proportion in Non-Malignant Cells Exacerbates Photon Emissions: Implications for Tumor Growth and Metastases. Int J Cancer Res Mol Mech 1(2): doi http://dx.doi.org/10.16966/2381-3318.108
Copyright: © 2015 Karbowski LM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
Received date: 01 July 2015
Accepted date: 01 August 2015
Published date: 04 August 2015
Abstract
Aim: Discern if there is a specific proportion of mixture of normal and malignant cells that increase photon emissions from cell cultures.
Method: Different proportions of B16-B6 mouse melanoma cells and HEX cells were mixed and allowed to proliferate. Photon emissions were measured from the different mixtures of cells by digital photomultiplier units and then analyzed for Spectral Power Density (SPD).
Results: Mixtures of the malignant and non-malignant cells that were more than 10% of one component displayed photon emission flux densities that were significantly less than the photon emissions for either cell type when measured as pure samples. Only 1% proportion of the malignant cell in 99% of non-malignant cells produced the strongest photon emissions. Spectral density profiles of power flux density variations indicated elevated power around 22 Hz that was even greater than this signature for malignant cells only.
Conclusion: Only 1% of malignant cells in a normal aggregate, representative of the early stages of cancer development, resulted in conspicuously increased numbers of photon emissions and spectral power spectra that often reflect a total malignant cell population. This combination of photon flux density and spectral power profiles may be a potentially useful (nanotechnology) tool to detect the minute changes in cell activities relevant to oncology.
Keywords
Cell proliferation; Photon emissions; Mixed cell populations; Malignant proliferation; Melanoma; HEXs
Introduction
All living organisms emit photons [1,2]. Several authors [1,3,4] have
shown that the emissions of ultra weak photons from basic biological
units such as cells and bacteria may convey the essential “information”
or “communication” patterns between these units. The temporal patterns
within amplitude fluctuations of photon emissions may serve as the “code”
or “key”, involving very little energy that determines the activation of the
massive molecular components which progress through pathways that
contain their own energies. From this perspective the ultraweak photon
patterns between cells would initiate intracellular process but likely not
affect them once they have been activated unless the light patterns are
coherent with more energetic sources such as pattern-synchronized
magnetic fields [5].
That the molecular pathways themselves are strongly correlated with
dominant frequencies between the ultraviolet through the visible to the near
infrared range has been shown by Dotta et al. [6]. They found that during
the ~ 20 hr after melanoma cells had been removed from incubation the
peak wavelengths of emitted photons changed from infrared to ultraviolet
which reflected the shifts from the initial activation of signaling molecules
(near-IR) to growth and protein-structure factors (near-UV). The specific
wavelength that was emitted from the corresponding molecular pathways
was predicted by Cosic’s Molecular Resonance Recognition equation
[7,8]. Filters with narrow transparencies of about 10 nm increments for
photon emissions over the PMT aperture were employed to verify Cosic’s
model. Photon emissions within predicted wavelengths were either
blocked or enhanced by the addition of pharmacological compounds that
either inhibited or facilitated specific pathways.
Although there have been recent concerns about intrinsic contamination
of cell lines [9], most cell culture experiments focus upon a single cell
line. Within the organism and its organs there are multiple cell types
existing simultaneously and proximally. The intrusion of malignant cells
into a population would begin with one or a few cells. Here we present
experimental results that when malignant and non-malignant cell lines
are mixed in different proportions markedly elevated photon emissions
occurred when the malignant cell was only 1% of the other cell line’s
population but not when the ratios were higher.
Methods
Mouse melanoma cells (B16-B6) and HEX cells were split from
confluent populations according to our usual procedures [10]. They were
combined into volumes of 10 mL in 50 mL centrifuge tubes according to
the following proportions: 100% B16; 100% HEX; 90% B16, 10% HEX;
50% B16, 50% HEX; 95% B16, 5% HEX; 90% B16, 10% HEX; 90% HEX,
10% B16; 95% HEX, 5% B16; 99% HEX, 1% B16. There were 6 replications
per group for a total of 48 preparations (and measurements).
The cells were re-suspended with 5 cc of additional media; 2.5 mL from
a given source were placed on tissue dishes (55 mm diameter) and allowed
to adhere for 24 hr to achieve 90% confluence. The plate was then placed
over the aperture of Digital Photomultiplier Unit (PMT). The PMT was
a SENS-TECH, Ltd, DM0089C model (370 to 680 nm band) with dark
counts of less than <40 photons per second. The numbers of photon counts
per 20 ms were obtained for 100 s (5000 samples). All measurements with
the PMT were completed within the incubators that were hyper-dark and
at standard temperature (37°C). We employed the same sampling and
measurement procedure utilized for measuring photon emissions from whole mice with and without tumors or from homogeneous malignant or non-malignant cells lines [11].
Spectral Power Densities (SPD) were obtained by SPSS-16 PC software
using methods described previously [12]. The sampling rate was 50 Hz (20
ms bins) due to the limits of the software and the sampling time was 100s.
The Nyquist limit was 25 Hz, that is, this sampling procedure allowed
discernment of amplitude variation frequencies >0 Hz to 25 Hz.
Results
Figure 1 shows the means and standard deviations for the numbers of
photons per 20 ms from the various mixtures of cell cultures. The values
are based upon 6 replications per condition. The B represents the B16
cells and the H refers to HEX cells. The number refers to the proportion
or percentage of each type of cell. When the proportions of malignant
and non-malignant cells were equal or there were 10% malignant cells in
90% normal cells or 10% normal cells in 90% malignant cells the photon
emissions were lowest. These photon emissions were significantly less
than the values when only B16 or HEX cells were homogenous (100%).
The strongest photon emissions occurred when the non-malignant (99%)
line contained only 1% of malignant cells.
The results of the spectral analyses are shown in Figure 2. The Critical
Spectral Power Density (SPD) is the 22 Hz amplitude band. This peak has
discriminated between different strains of human and animal malignant
cell lines and non-malignant cells. It was significantly elevated for the
mixed populations within the highest disproportions of cells. Although
the elevation of 22 Hz band SPD for 100% B16 cells compared to 100%
HEX cells was expected, the power density was more than doubled in the
mixtures where the B16 cells were composed 1% to 10% of the population.
When the proportions of the two cell lines were equal or if 10% of the
population was composed of HEX cells (90% melanoma) the density
values did not differ significantly from a homogeneous population of
normal cells.
Discussion
To our knowledge this is the first experimental demonstration that
different proportions of mixtures of cells, more typical of tissue, produce
different radiant flux densities of photon emissions. Equal proportions of
two lines, one malignant, one not-malignant, or mixtures when there was
10%:90% proportions emitted about one-third of the photon power density
from dishes that contained 100% of either of the cells. This suggests that
once the proportion of one cell line is 10% or more within the aggregate
the photon emissions diminish significantly. In other experiments involving adjacent plates of microtubule preparations [13] such decreases
in photon emissions have strongly suggested enhanced exchange of
photons between the two preparations of microtubules. If this process was
occurring here, then once the proportion of the other cell exceeds 10%
the numbers of photons emitted into the general environment decreases
and more of the photon emissions would remain intercellular. This would
be consistent with intra-unit “communication” roles of photons proposed
by Fels [3] and Trushin [4]. However it may also indicate a non-specific
decrease of the processes that generate the intercellular photon emissions.
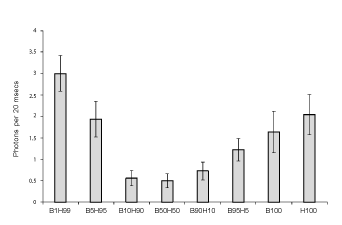
Figure 1: Numbers of photons per 20 ms as a function of the proportion of cell line mixtures were B is B16 (malignant) cells and H is HEX (nonmalignant) cells. B100 and H100 refer to homogeneous populations of each type of cell. Vertical lines indicate standard deviations.
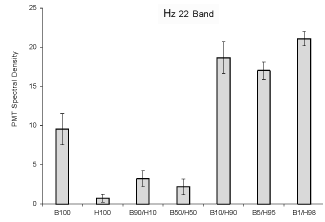
Figure 2: Relative units of Spectral Power Density (vertical axis) for photon emissions for the amplitude enhanced at the 22 Hz band as a function of the various combinations of malignant and non-malignant cells. Vertical lines refer to standard deviations.
By far the most novel and potentially important result from the
perspective of “metastasis” processes was the marked elevation of photon
emissions when only 1% of the non-malignant cell population contained
malignant cells. This enhanced photon density was about double the
values of the homogeneous populations and 6 times the power for different
proportions of the cells. The functional power flux density from the 1%
contamination would be equivalent to (assuming 5·10-19 J per photon and
50, 20 ms increments per s) 7.5·10-17 W. Because the aperture was about
2 cm2, the power density would be about 0.4·10-12 W·m-2. If the spherical
radiation of the photons is accommodated the actual power density would
be around 2.5·10-12 W·m-2. This is within the range of power density emitted
by malignant cells lines that have been “distressed” by removal from 37°C
incubation and maintained at room temperature [11]. On the other hand
the typical background would be close to 2.5·10-13 W·m-2 which is within
the range of background cosmic radiation at sea level.
This increase in photon emission from the 1% “contamination” would
also be within the range of the spikes of photon emissions recorded from
melanoma cells following injections of therapeutic dosages of morphine
[14]. Morphine compounds have been associated with metastases [15].
One interpretation of our results is that the initial intrusion of the
malignant cell into a normal cell environment is associated with marked
increases in photon emissions that could serve as stimuli for other cells to
respond, like an “alarm”. Alternatively the photon emissions may reflect
information that would contribute to the migration of other malignant
cells into the location or to the transformation of parenchymal cells
into malignant forms. De-differentiation of cell types into those with
malignant potential may be more frequent than suspected. If photons are
involved with the mediation of “information” one would expect subsets or
classes of “communications” that could facilitate a range of functions that
include (metaphorically) “alarm”, “cooperation” or even “submission” to
the cellular context.
From a diagnostic perspective the spectral power densities are revealing.
Although the amounts of photons have been considered the essential
measurement for discerning the presence of tumors [16], our analyses here
and in other contexts [12] indicate that the spectral power density within
the extremely low frequency range has greater differentiating potential. In
the present study the classic power fluctuation frequency (22 Hz) in cancer
cell lines we have noted frequently during photon measurements was
doubled in the normal cells with 1%, 5% and 10% mixtures of melanoma
cells. When the value reached 50%-50%, this “abnormal” pattern was not
observed. The source of the 22 Hz enhancement must still be isolated.
For example, as suggested by reviewers, comparisons of the results from
an immunocytochemical stain for S-100 protein Ab for the co-culture of
1% B16 cells mixed with 99% HEX cells and the 50%-50% combination
at the 24-hr point after passage could discern relative plating of B16 cells.
Consequently the association between viable (plating) of B16 cells and the
SPD amplitudes of the 22 Hz peak could be determined. However even
without such differentiation, the results support our suggestion that these
profiles of photons might be developed to discern very early tumor growth
or malignancy before it is even apparent by more traditional methods
of imagining and identification when the tumor is very large and more
difficult to treat.
Summary at a Glance
Different proportions of mixtures of cell lines, in this case a malignant
and non-malignant cell type, emitted statistically significant different
numbers of photons. Only 1% “contamination” of the normal cell line by
the malignant cell generated marked increase in photon emissions from
the aggregate of cells. The Spectral Power Density (SPD) profiles were
similar but stronger than those from aggregates of homogeneous (100%)
malignant cells. Photon (irradiant) flux density and SPDs in combination
might be employed to discern the first stages of infiltration into or
development of malignancy in healthy organs.
Conflicts of Interest
NJM, LMK, BTD and MAP have no potential conflicts of interest to report.
Acknowledgement
Thanks to Dr. WE Bosarge, CEO of Capital Technologies, Inc. for supporting this research.
Download Provisional pdf here



