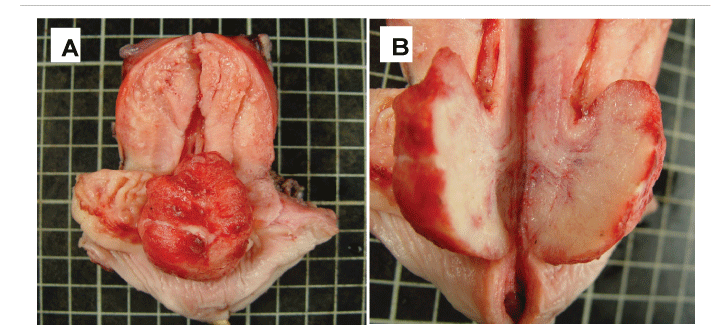
Figure 1: (A) Gross photograph shows a polypoid tumor arising from posterior aspect of endocervical canal.
(B) Grossly, the tumor reveals elastic in consistency, and light tan in color on cut surface.

Chi-Min Shih1 Chun-Shuo Hsu2,3*
1Department of Pathology, Chia-Yi Hospital, Ministry of Health and Welfare, Chia-yi, Taiwan*Corresponding author: Chun-Shuo Hsu, Department of Obstetrics and Gynecology, Buddhist Dalin Tzu Chi General Hospital, 2, Min Sheng Road, Dalin Township, Chiayi, 622 Taiwan, TEL : 886-5-264-8000 ; Ext : 5240 ; Fax : 886-5-264-8006; E-mail: A123014@tzuchi.com.tw
Article Type: Case Report
Citation: Hsu CS (2015) A Polypoid Cervical Neuroendocrine Carcinoma with Tumor Heterogeneity and Synchronous Squamous Intraepithelial Neoplasm and Adenocarcinoma In Situ. Int J Cancer Res Mol Mech Volume 1 (1): doi http://dx.doi.org/10.16966/ 2381-3318.104
Copyright: © 2015 Shih Chi-Min, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
It is rare to have three synchronous neoplasms including neuroendocrine carcinoma, squamous cell and glandular neoplasia in a uterine cervix. Here, we present the case of a 35-year-old woman with Cervical Polypoid Neuroendocrine Carcinoma (NEC), High-Grade Squamous Intraepithelial Lesion (HSIL), and Endocervical Adenocarcinoma In Situ (AIS). DNA in situ hybridization of high-risk HPV (types 16, 18, 31, 33, and 51) DNA shows more intense signals over the HSIL and underlying Squamous Intraepithelial Lesion (SIL)-related atypical endocervical glands and less intensity over AIS and NEC. The heterogeneity of NEC is exhibited by various immunohistochemical staining patterns of chromogranin A, cytokeratin 7, CD56, CD44, c-kit, and vimentin, as well as histomorphology. CK7 immunostaining is observed over HSIL and AIS, CK5 over HSIL and underlying SIL-related atypical glands, P63 over HSIL, and Hep Par1 over AIS. In conclusion, this cervical NEC with tumor heterogeneity has unusually polypoid gross feature and fatal outcome. She died due to metastatic neuroendocrine carcinoma 21 months later. The special characters of SIL-related atypical endocervical glands, which show CK5 immunostaining, loss of expression of estrogen and progesterone receptors, and intense HR-HPV DNA hybridization, disclose the discrepancy between morphology and CK5 immunostaining result, which might be an early evidence of neoplastic transformation.
Cervical AIS; High-grade SIL; Cervical neuroendocrine carcinoma; HPV in situ hybridization; IHC study
Small cell carcinoma of uterine cervix is rare. The simultaneous presence of three neoplasms, including neuroendocrine, squamous cell, and glandular neoplasms, in a uterine cervix is also rare, although two different neoplasms occasionally may be seen simultaneously [1,2]. Here we report a case of triple cervical neoplasms: Neuroendocrine Carcinoma (NEC), High-Grade Squamous Intraepithelial Lesion (HSIL), and Adenocarcinoma in situ (AIS). The etiologies of cervical epithelial neoplasms are associated with various types of Human Papilloma Virus (HPV). HPV types 16 and 18 are well known to be associated with squamous cell carcinoma and other types of epithelial neoplasms of the uterine cervix [3-6], and HPV type 52 is also prevalent in Taiwan [7]. We are interested in the detection of high-risk (HR)-HPV by means of DNA In Situ Hybridization (ISH), and in performing Immune Histo Chemical (IHC) studies to characterize this unusual polypoid neuroendocrine tumor and its additional types of cervical neoplasms [1,2,8-10] and following up the outcomes of these cervical tumors after radical hysterectomy and concurrent chemoradiotherapy.
A 34-year-old woman, gravida 2, para 2, had episodic postcoital vaginal bleeding for 1 month. She had not had Pap smear for 10 years. Pelvic examination revealed an exophytic mass with superficial erosion over the cervix nearly 4 cm in maximum dimension, with no parametrial involvement. Pelvic computed tomography revealed that the parametrium and perivesical and perirectal spaces were preserved. There w ere n o abnormally enlarged lymph nodes over the parailiac regions bilaterally or in the para-aortic area, and colonoscopic and cystoscopic findings were non-specific. Clinical stage was 1B1 (AJCC 2010).
Laboratory test results were within normal limits, and tumor markers showed Carcinoembryonic Antigen (CEA): 1.43ng/ml, cancer antigen 125 (CA125): 7.2U/ml, and Squamous Cell Carcinoma (SCC) antigen: 1 ng/ml.
Radical hysterectomy and bilateral pelvic lymph node dissection were performed. Grossly, a 4.2 x 3.2 x 1.6 cm polypoid tumor was observed, arising from the posterior wall of the endocervical canal (Figure 1).
Microscopically, in addition to exophytic neuroendocrine tumor with intermediate sized, pleomorphic ovoid nuclei, and the tumor cells arranged in solid nests with focal vascular invasion over the endocervix, there were non-keratinizing HSIL over the transformation zone and AIS in between the NEC and the HSIL (Figure 2A-2D). Except for reactive hyperplasia of pelvic lymph nodes bilaterally, no other remarkable findings were noted in the tissue specimen.
IHC and ISH studies were performed on a Leica BOND-MAX autostain machine accompanied by negative and positive control tissue sections in each batch. The IHC studies for NEC show diffusely positive immunostaining for synaptophysin and cytokeratin AE1/AE3 (CK), and focal immunostaining for cytokeratin 7 (CK7), chromogranin A, CD56, CD44, CD117 (c-kit), and vimentin, and are negative for CK5, estrogen receptor (ER), progesterone receptor (PR), and Her-2/neu. Ki-67 proliferation index is markedly elevated to > 50% over NEC and AIS and moderately elevated to around 50% over HSIL. The tumor heterogeneity is observed with the variable immunostaining patterns in the NEC. At least four different portions are recognizable as: DSP - distal superficial portion, DDP - distal deep portion, PSP - proximal superficial portion, and PDP - proximal deep portion with somewhat variable histomorphologic patterns (Figure 2E-2H). Immunostaining results, excluding the negative results of TTF-1, HER-2/neu, ER and PR on entire tumor cells, are all listed in the Table 1. Notably, CK7 is also expressed over endocervical glands, AIS, and HSIL. CK5 and P63 are positive over benign squamous epithelium and HSIL, but CK5 is still immunostained over SIL-related atypical glands beneath the HSIL lesion. Hep Par1 is positive only over AIS.

Figure 1: (A) Gross photograph shows a polypoid tumor arising from posterior aspect of endocervical canal.
(B) Grossly, the tumor reveals elastic in consistency, and light tan in color on cut surface.
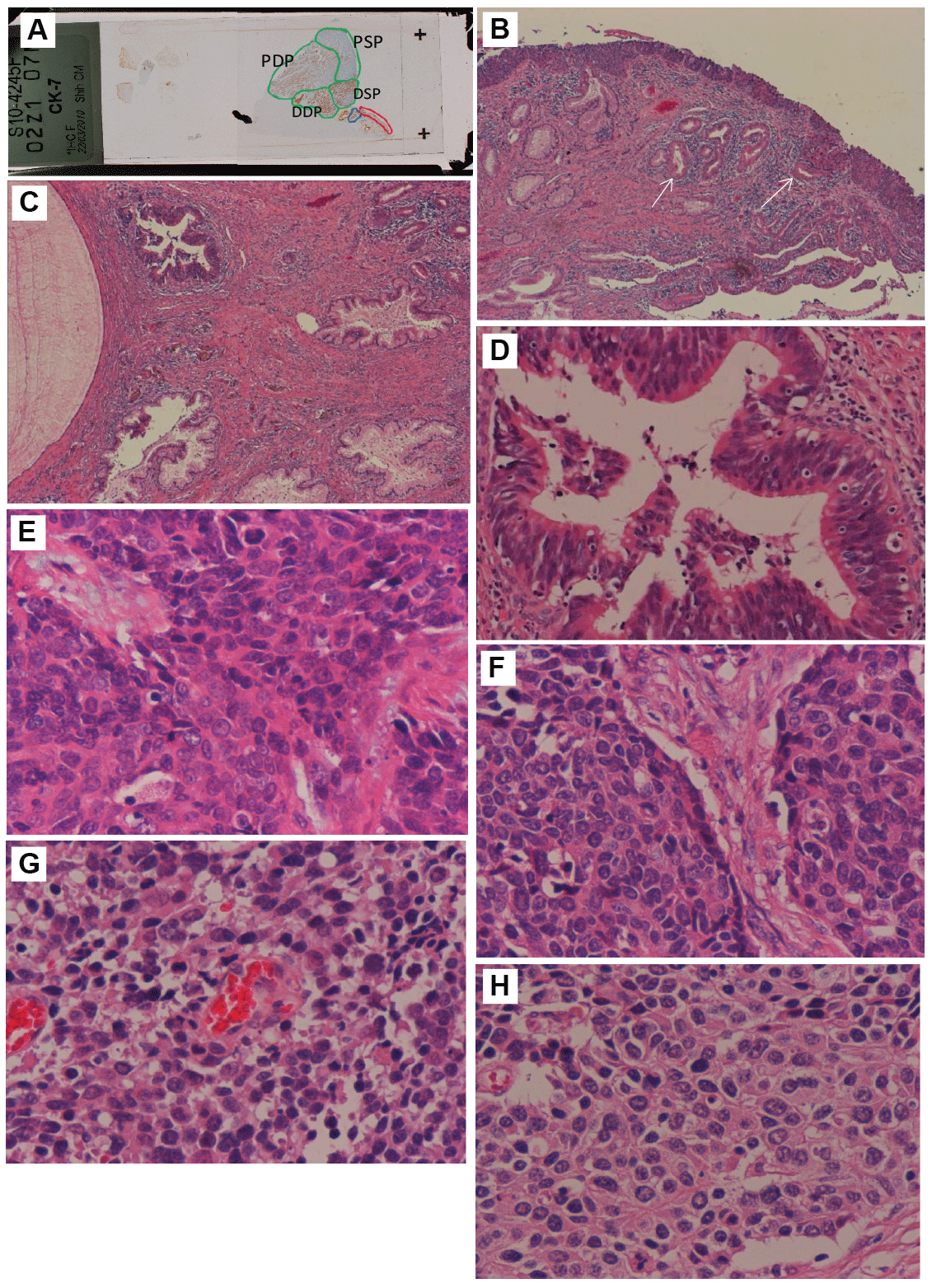
Figure 2: (A) Scanning view of CK7 immunostaining displays squamous cell HSIL (encircled by red line), endocervical AIS (encircled by blue line), and exophytic NEC (encircled by green line) which can be roughly divided into 4 portions by immunostaining and histomorphologic patterns.
(B) HSIL is remarkable, and the superficial glands beneath HSIL are SIL-related atypical glands (arrows)(hematoxylin & eosion, original magnification 40x).
(C) AIS over left upper area of the photo (hematoxylin & eosion, original magnification 40x). (D) High power view of the AIS (hematoxylin & eosion, original magnification 200x). (E-H) The 4 main different histologic patterns of NEC are shown as
(E) distal superficial portion,
(F) distal deep portion,
(G) proximal superficial portion, and
(H) proximal deep portion (hematoxylin & eosion, original magnification 400x).
The variable intensities of HR (types 16, 18, 31, 33, and 51)-HPV DNA ISH signals demonstrate HSIL and SIL-related glands > AIS > NEC. The HR-HPV DNA ISH shows abundant intense larger dots over the squamous cell CIS and underlying SIL-related atypical glands (Figures 3A and 3B), but less intensity and smaller dots over AIS (Figure 3C), and over NEC (Figure 3D) with variable signal intensities of DSP > DDP > PSP > PDP. In situ hybridization of HPV type 6 and 11 DNA was completely negative.
Following radical hysterectomy, the patient received concurrent chemoradiotherapy with etoposide (100 mg/m2 ) and cisplatin (70 mg/m2 ) every 3 weeks in the Hematology and Radiation Oncology departments. The patient did well for 15 months until she was found to have a metastatic NEC in left kidney, and received left nephrectomy subsequently. Unfortunately, she expired 6 months after the left nephrectomy
Although the literature describes HPV type 18 as being detected more frequently in NEC than is HPV type 16 [6,7,11], in our case, HR-HPV signals are more intense on the HSIL, and less intense in AIS and NEC. HPV type 16 infection is still a possibility for this case, since HPV type 16 is sometimes detected in NEC [3,5].
Synaptophysin was diffusely positive over NEC, whereas chromogranin A was focally positive, as the IHC staining results were described by Ishida et al [2]. In addition to synaptophysin +++, DSP of this NEC also reveals chromogranin A+, CD56++ and CK7+++, showing more obvious neuroendocrine differentiation than did other portions of this NEC tumor. CD44, an adhesion molecule in cell-to-substrate and cellto-cell interaction, is present over the cytoplasm of focal HSIL and focal NEC, that is completely absent over DSP of NEC, or AIS (Table 1), and somewhat compatible with the study of Kuo et al. [10], the reports of CD44 expression in 47 squamous cell carcinomas (47/50), 14 adenocarcinomas (14/28), and only one neuroendocrine carcinoma (1/17). CK7 has markedly positive staining over the normal endocervical glands, AIS, distal portion of NEC, and especially on cervical squamous cell neoplasm as were reported by Chu P et al. [12]. CK7 immunostaining on the cervical HSIL suggests that it should be not purely squamous cell differentiation. C-kit, a receptor tyrosine kinase of hematopoietic stem cells may have activating mutations in several tumors, is present over basal layer of HSIL, and only over deeper portion of DSP of NEC. The DD portion of NEC showing irregular solid nest pattern and a little bit denser cytoplasm is morphologically more similar to high-grade cervical squamous cell carcinoma than that of other portions, and is more like the cancer cells of vascular invasion site. PSP and PDP of NEC, with the lowest density of HR-HPV signals, have relatively lower N/C ratios (Figures 2G and 2H), less immunostaining intensity of CK7, and spotted areas of necrosis in focal tumor lobules (not shown in Figure 1), being more approaching the character of atypical carcinoid tumor.
Adenosquamous carcinoma may be found in lung or in the uterine cervix. CK7 and high molecular weight CK5 are useful tools to differentiate between adenocarcinoma and squamous cell carcinoma in the lung. However, the CK7 is more readily immunostained over non-glandular genitourinary neoplasms, such as urothelial carcinoma and cervical squamous epithelial cancer. CK5 is a marker to prove the squamous cell differentiation of not only HSIL with glandular involvement but also its beneath SIL-related atypical endocervical glands (Figure 4A), which have a single layer of irregularly enlarged nuclei, eosinophilic cytoplasm, intense HR-HPV DNA hybridization (Figures 3A and 3B), loss of ER and PR, and moderate increase of Ki-67 proliferation index. P63 marker is related to myoepithelial cell, basal cell, or stratified squamous cell differentiation13, and is mainly immunostained over lower layers of HSIL lesion and stratified squamous epithelium but not over SIL-related atypical endocervical gland. Therefore, the pathologic diagnosis of atypical oxyphilic metaplasia [14] or suspicious precursor of squamous epithelial neoplasm should be considered. AIS has branching glandular structure, crowded elongated nuclei, the IHC findings of Hep Par 1 immunoreactivity (Figure 4B), and marked increase of Ki-67 proliferation index. The staining of Hep par1 highlights that the presence of AIS glands is compatible with the finding of TP Thamboo et al. [9]. The SIL-related atypical endocervical glands just beneath the squamous epithelial neoplastic lesion with positive CK5 and negative Hep Par 1 immunostainings as in this case, should not be mistaken as a glandular neoplastic lesion.
ER and PR, which are present over the basal and parabasal layers of squamous epithelium and endocervical glands, are absent over the HSIL, SIL-related atypical glands, AIS or NEC during neoplastic transformation.
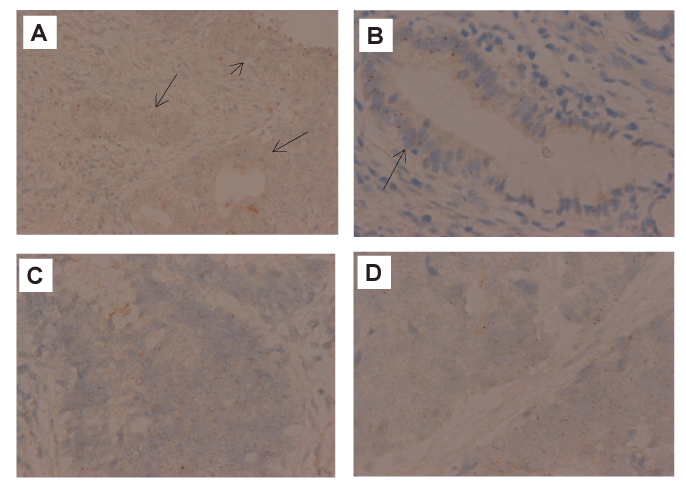
Figure 3: (A) HR-HPV DNA ISH shows HSIL (short arrow) and its underlying SIL-related atypical glands (long arrows) having strong hybridization signals (200x).
(B) High power view reveals remarkable HR-HPV DNA signals over SIL-related glandular portion (left half of the gland)(arrow), and no hybridization signal over normal glandular portion (right half of the gland) (400x).
(C) AIS lesion reveals appreciable intensity of HR-HPV DNA signals (400x).
(D) Visible intensity of HR-HPV DNA signals over NEC are noted. (400x).
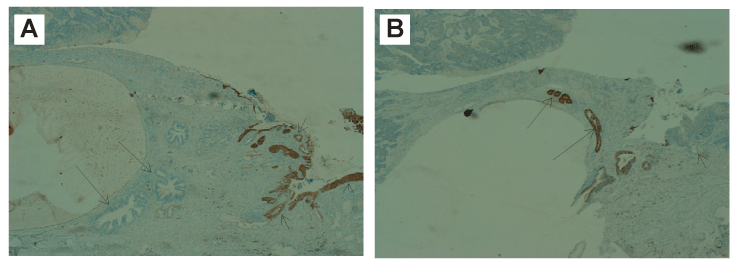
Figure 4: (A) Positive CK5 immunostaining over HSIL (arrow head) and SIL-related glands (short arrows), and negative over AIS lesion (long arrows) (40x).
(B) Hep Par1 IHC stain demonstrates positive staining over AIS lesion (long arrows), but negative staining over benign endocervical glandular epithelium or SIL-related atypical glands (short arrow) (40x).
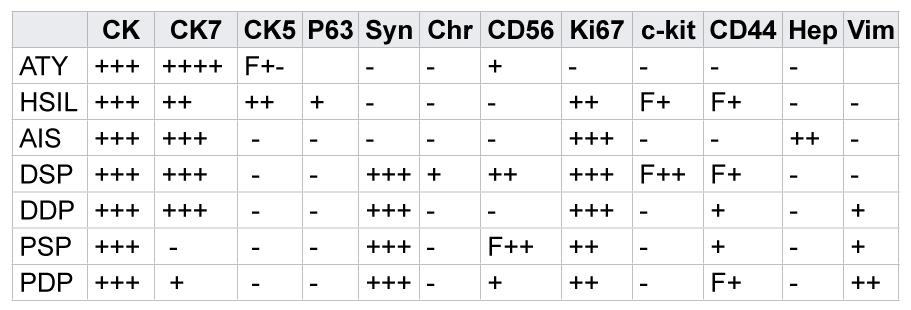
ATY=SIL-related atypical gland;
DSP=distal superficial portion of neuroendocrine carcinoma (NEC);
DDP=distal deep portion of NEC;
PSP=proximal superficial portion of NEC;
PDP=proximal deep portion of NEC.
CK=cytokeratin AE1/AE3;
CK7=cytokeratin 7;
Syn=synaptophysin;
Chr=chromogranin A;
c-kit=CD117;
Hep=Hep-Par1;
Vim=vimentin;
F=focal; +++=strong staining;
++=intermediate staining;
+=weak staining.
Note: Chromogranin A and c-kit immunostaining are positiveonly over the DSP of NEC, which, exceptionally, has loss of CD44 expression.
Table 1: The immunostaining results of various tumor markers
Small cell carcinoma or large cell neuroendocrine carcinoma of the uterine cervix has a poorer prognosis than does non-small cell carcinoma [11,15,16]. After adequate treatment, the patient of polypoid neuroendocrine carcinoma may have a better prognosis, as suggested by few reports of a few cases [17,18]. This polypoid neuroendocrine carcinoma with tumor heterogeneity and vascular invasion behavior turns out to have worse prognosis.
We would like to thank the contributions of qualified laboratory results from the Department of Pathology of Buddhist Dalin Tzu Chi General Hospital.
Download Provisional pdf here
All Sci Forschen Journals are Open Access