Abstract
Aim: Assess timeliness of lung cancer management, causes for delays, and whether length of delays affected the prognosis.
Method: A retrospective study of patients diagnosed with lung cancer between January 2012 - September 2014.
Results: The median (range) delay in CT scan from first presentation to health care setting, “CT scan-to-first diagnostic procedure,” “first
diagnostic procedure to confirmed diagnosis,” “confirmed diagnosis-to-start of treatment” was 13 (1-399), 7 (1-490), 3 (1-176), and 35 (1-150)
days respectively. The median length of the journey from “CT scan-to-start of treatment,” and “first presentation to healthcare-to-start of treatment”
was 56 (6-192), and 74 (2-438) days respectively. Thirty six percent waited more than 2 months to start definitive treatment from the time of their
CT scan. Less-timely care correlated with those who underwent transthoracic needle aspiration, elderly males, and had non-small cell carcinoma.
It also correlated with better survival 272 (18-965) vs. 97 (2-1615) days (p=0.01) due to more number of early stage lung cancer in this group
(43.1% vs. 27.1%, p=0.05). Common causes of less-timely care were misdiagnosis of cancer as TB, failure of first diagnostic procedure to provide
diagnosis, delay in patient`s decisions regarding initiation of therapy, and development of inter-current illness while waiting for therapy.
Conclusions: Delay in the management of early stage lung cancer patients was seen. CT guided biopsy (transthoracic needle aspiration),
advanced age, male gender, and NSCLC were the predictors of delay. Limited (twice weekly) availability of CT guided biopsy, misdiagnosis as
TB, delayed patients` decision, and development of inter-current illness were the main causes of delay. Correlation between less timely care
and better survival, attributable to the early stage indicates risk for progression of the disease and merits measures for more efficient resource
allocation.
Keywords
Timeliness; Bronchoscopy; Cancer (lung); Tuberculosis
Introduction
Journey of the suspected lung cancer patients in the hospital is made
up of four sequential stages. The timeliness of each stage depends on the
timeliness of the output of the stage prior to it. These stages are: 1) first
presentation to healthcare facility for clinical & radiographic features
of lung cancer-to-first computed tomography (CT) scan or pulmonary
specialist consult; 2) CT scan-to-diagnostic procedure; 3) diagnostic
procedure-to-confirmation of diagnosis, and 4) confirmation of diagnosisto-
start of treatment.
It is intuitively conceivable that minimizing delay in these stages will
translate into quicker diagnosis, early initiation of treatment, and better
outcomes. Correspondingly several guidelines have been established
setting target intervals for maximum wait in each stage. Based on the
recommendations of Swedish Lung Cancer Study Group, most patients
with suspected lung cancer should complete the diagnostic test by 4 weeks
of consulting the chest physician [1]. This should be followed by initiation
of therapy within 2 weeks [1]. The guidelines from UK recommend
initiation of radical radiotherapy within 2 weeks of requesting it [2].
Similar time frame have been proposed by the guidelines from Canada [3].
In general, the maximum wait time permissible between first presentation
to healthcare facility for clinical & radiographic features of lung cancer
and start of treatment is 60 days [4,5].
However, the reports on the impact of the timeliness of care on
prognosis in the published literature provides conflicting results. In
systematic review by Olson et al, no association between timeliness
and outcome was seen in 8 studies [6-13]. Some studies showed inverse
relationship between survival and delay in diagnosis and treatment [14-
16], and some studies paradoxically showed favourable relationship
between delay and survival [17-21].
We did this study to assess timeliness of lung cancer management,
and causes for delays. We also evaluated whether length of delays were
acceptable, and examined their relationship with prognosis.
Methods
This is retrospective evaluation of lung cancer patients who were
managed by our pulmonary department between January 2012 and
September 2014. Data was collected on demographics, Computed
Tomography (CT) findings, type of diagnostic technique employed,
pathological result, number of procedure required to reach conclusive
diagnosis, and time from first presentation to healthcare facility for
clinical & radiographic features of lung cancer-to-first CT scan, CT
scan-to-diagnostic procedure, diagnostic procedure-to-confirmation of
diagnosis, and confirmation of diagnosis-to- start of treatment. Approval
from Institutional board review was obtained.
Definitions
Timely care
Patient were considered to have received timely care if the duration
between their CT scan and start date of treatment (chemotherapy
radiotherapy, surgery or tyrosine kinase inhibitors) was 60 days or less
[4,5].
Less timely care
Patient were considered to have received less-timely care if the duration
between their CT scan and start date of treatment (chemotherapy,
radiotherapy, surgery or tyrosine kinase inhibitors) was more than 60
days.
Data analysis
We used software (SPSS, version 17; SPSS, Chicago, Ill) for all statistical
analyses. The results were compared using a Wilcoxon two-sample test or
Fisher exact test. P values were two sided and considered indicative of a
significant difference if less than .05.
Results
Out of 202 patients, 82 (41%) had adenocarcinoma, 29 (14%) squamous
cell carcinoma, 13 (6%) small cell lung cancer (SCLC), 11 (5.4%) nonsmall
cell cancer, and 67 (33%) had other sub-types. Forty three were
treated with chemotherapy, 44 with radiotherapy (RT), 21 with surgery,
32 with tyrosine kinase inhibitors (TKI), 1 with laser therapy, 5 patients
were treated at another hospital, 5 defaulted, 10 declined, 6 died before
treatment, 1 patients was undecided, and 34 received best supportive care,
[Table 1].
Timeliness of care
The median (range) length of the journey from “1st presentation to
healthcare-to-start of treatment” was 74 (2-438) days.
1st presentation-to-CT scan: The median length of time between
1st presentation-to-CT scan for all patients was 13 (1-399) days. Fifteen
(20.5%) of patients waited more than 2 weeks for CT scan from the time of
1st presentation with features of lung cancer. After developing lung cancer
related symptoms, patients who first presented to Emergency Department
(ED) had shorter delay of 2 (1-232) days in having the CT scan performed
as inpatient during their hospitalization, versus those who presented to
General Practitioner (GP), poly-clinic, or non-respiratory physician clinic
of 5 (1-595) days (p=0.02). Those referred to respiratory specialist and
managed either as suspected lung cancer (n=30), or as suspected smear
negative tuberculosis (n=24) had significantly longer delay in performing
the CT scan of 6 (2-201), and 28 (2-438) vs. 2 (1-595) days for those
referred to ED (p=0.003) respectively. Patient initially managed as smear
negative tuberculosis had longest delay in this stage [Table 2].
CT scan-to-first diagnostic procedure: The median length of time
between CT scan-to-first diagnostic procedures was 7 (1-490) days. Sixty
seven (34.5%) had delay of more than 2 weeks between “CT scan-to-first
diagnostic procedure.” Main reasons were patient refusal and missed
radiographic opacity
First diagnostic procedure-to-diagnosis: The median length of
time between 1st diagnostic procedure (thoracentesis, pleural biopsy,
bronchoscopy, percutaneous biopsy, or others)-to-confirmation of
diagnosis in 181 patients was 3 (1-176) days. Two hundred and forty one
procedures (1.19 per patient) were done in 202 patients. Twenty four
(13%) had a delay of more than 2 weeks in this stage. Main reason for
delay was need for multiple procedures to establish the diagnosis due to
non-diagnostic first procedure. Forty six (22.7%) patients had a mean
delay of 13 days between “first diagnostic procedure-to-diagnosis” due
to need for 2 or more procedures compared to 2 days in those requiring
single procedure (p= 0.004).
Diagnosis-to-start of treatment: The median length of diagnosisto-start
of treatment time in our cohort was 35 (1-150) days. Sixty eight
(48.9%) patients had a delay of mean of more than 1 month. Longest delay
of more than 6 weeks occurred in nearly a third (40/139, 29%) of patients
in this stage. Main reasons were awaiting patient`s decision, development
of inter-current illness, waiting for staging, lung tumour board, surgery,
and mutation analysis result.
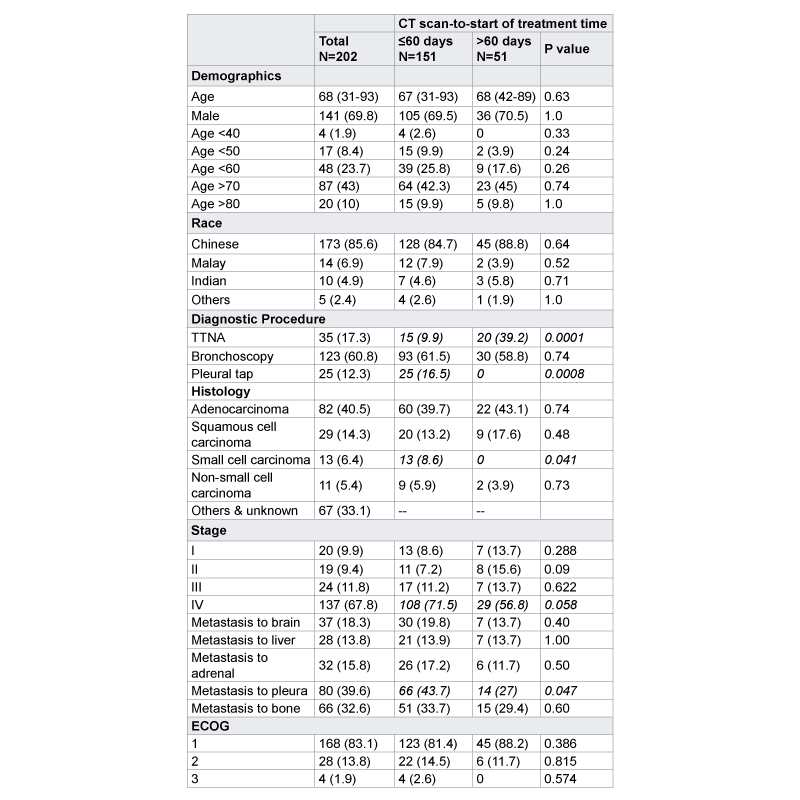
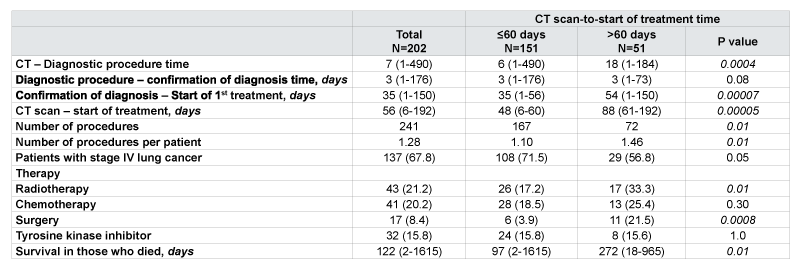
Data presented as number (%) or mean (±SD)
TTNA: Transthoracic needle aspiration; ECOG: Eastern Cooperative Group.
Table 1: General characteristics of patients with subgroup analysis of
timely CT scan-to-start of treatment time (≤60 days) and less-timely CT
scan-to-start of treatment time (>60 days) groups (n=202).
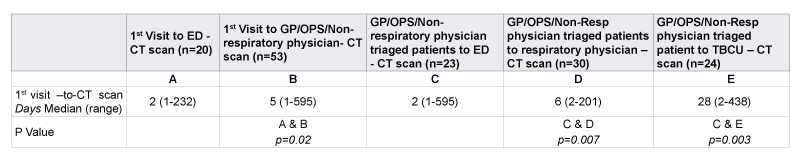
Data presented as number (%) or mean (±SD)
ED: Emergency department; CT: Computed tomography; GP: general practitioner; OPS: poly clinic; TBCU: TB control unit.
Table 2: Timeliness of the first presentation-to-CT scan stage
RAND Corporation targets a maximum interval of 8 weeks from chest
x-ray or CT scan of the chest showing mass or nodule and its surgical
resection [22]. The mean length of CT scan-to-treatment interval was 56
(6-192) days. Fifty (36%) waited more than 2 months to start definitive
treatment from the time of their CT scan, [Figure 1]. The reasons for the
delay in each stage are presented in Table 3.
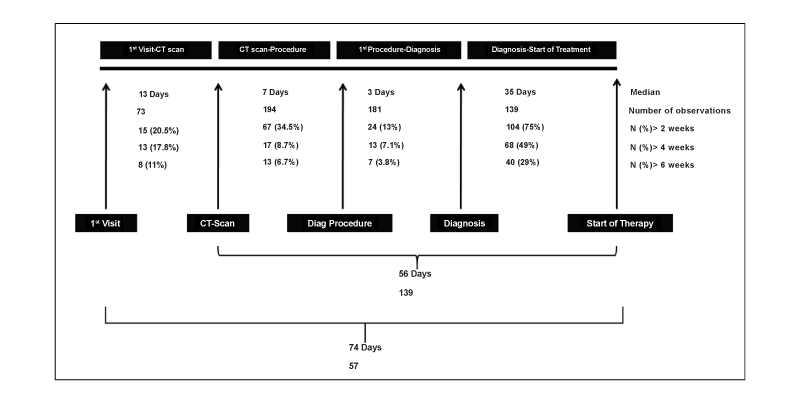
Figure 1: The median delays, and the number of observations in the diagnostic workup.
Predictors of timely care
Factors associated with less-timely care in univariate analysis were lack
of pleural effusion, Transthoracic Needle Aspiration (TTNA), sub-type
other than small cell carcinoma, curative surgery, radiotherapy, greater
number of diagnostic procedures, and initial treatment as smear negative
tuberculosis. Multivariate analysis revealed TTNA, sub-type other than
small cell carcinoma, advanced age, and male gender as factors associated
with less-timely care.
Timeliness of care and prognosis
Median survival was 122 (2-1615) days. Mortality showed negative
correlation with the timeliness. Despite prompt care, patients in timely
group had a shorter survival 97 (2-1615) days vs. less-timely group 272
(18-965) days (p=0.01) due to greater proportion of advanced stage lung
cancer patients in the timely group. Survival was greatest and significantly
higher in patients who underwent resection (early stage) versus those who
did not (late stage) with 459 (286-927) vs. 117 (2-1615) days, p= 0.0005)
respectively, [Table 4].
Discussion
The results of this study indicate that a third of patients were delayed
beyond the recommended time targets in each stage. Longest delay
affecting most number of patients was seen between confirmation of
diagnosis-to-start of treatment. However, as the delay occurred more
frequently in patients with early stage cancer, it was not associated with
poorer prognosis. This indicated patients pre-determined to have poor
prognosis by virtue of their advanced stage were being treated more
expeditiously for an unavailing benefit than those who could gain more
in-terms of survival from such expediency.
In the pre-diagnosis stage, upon their first presentation to primary
physician, some patients were suspected to have TB instead of cancer.
These patients had a greater delay in performing the CT scan as compared
to those referred to respiratory physician or ED. This may have been
due to regional prevalence of TB and inappropriate attribution of the
radiograph changes to smear negative TB. Fifty to 80% of patients with
pulmonary TB have positive sputum smears [23]. The remaining smear
negative patients in high prevalence countries often mislead clinicians to
diagnose lung cancer as TB due to clinical and radiological similarities of
pulmonary TB with lung cancer [24]. Main reasons for this error is the
delay in investigating the opacities detected on chest radiograph by CT
scan or Fibre Optic Bronchoscopy (FOB) [24]. Lack of utilization of these
tests in developing countries is attributed to their high cost and limited
availability limited to urban areas and tertiary care centres. Hence, in the
high TB prevalence areas, therapeutic trial of TB treatment is an acceptable
practice. However, therapeutic trial of TB treatment should be limited to
a certain period, beyond which the diagnosis of TB must be reconsidered
for poor or no response. A prospective case series of 107 patients of
cutaneous TB indicated that if patient does not respond to 5 weeks of TB
treatment, the diagnosis of TB should be reviewed [25]. Whether this can
be extrapolated to pulmonary TB remains to be established.
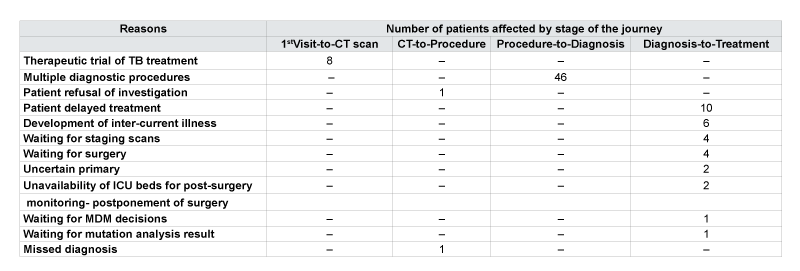
Table 3: Reasons for long delay in the 4 stages of the journey of lung cancer
Data presented as number (%) or mean (±SD)
Table 4: Subgroup analysis of timely (CT scan-to-start of treatment time of ≤ 60 days) and less-timely care (CT scan-to-start of treatment time of >60 days), by stage of cancer, therapy, and survival. Less-timely care correlated with early stage cancer, radiotherapy, surgical resection, and better survival.
Another common reason for the delay in the “pre-diagnosis” stage
(diagnostic procedure-to-confirmation of diagnosis) was failure of single
procedure to yield the diagnosis. These findings are similar to existing
literature. Need for multiple diagnostic tests and consultations has been
reported as common causes of delay by other investigators [5, 26]. British
Thoracic Society recommends that the results of bronchoscopy or any
other similar diagnostic test, including the histological or cytological
result, should be available within 2 weeks of a decision to do it [27].This
seems feasible as this time interval in our cohort was 2 days for those who
needed only single procedure and 13 days for those who needed more
than 2 procedures.
In the post-diagnosis stage, delay of average more than 1 month was
seen in diagnosis-to-start of treatment in half of the patients. The Swedish
Lung Cancer Study group recommends that treatment should be started
within 2 weeks after completion of diagnostic tests [1]. In the UK it is
advocated that radical radiotherapy should start within 2 weeks after it is
requested [2]. In Canada the recommended waiting time from completion
of diagnostic tests to surgery should not exceed 2 weeks [3]. The NHS
National Cancer Plan and RAND Corporation target a maximum interval
of 4 weeks and 6 weeks respectively from diagnosis to treatment. The
implication of this delay is that time observed for lung tumours to double
their volume ranges from 4 to 56 weeks, with a median time of 17 weeks
[28,29]. Although only a third (50 patients) had CT scan-to-treatment
time of more than 8 weeks, 12 patients in our cohort had the CT scan-totreatment
time of more than120 days (16 weeks). It seems likely that delay
of 16 weeks, which approximate to one tumour volume-doubling time for
Non-small Cell Carcinoma (NSCLC) in these patients would have made
some tumours inoperable.
The most common reason for delay in the “post-diagnosis” stage
was patient taking time to decide about their treatment, followed by
development of inter-current illness, and waiting for completion of
staging, treatment decisions from Multi-Disciplinary Meetings (MDM),
and surgery. Waiting time for surgery, and reluctance to undergo invasive
procedures has also been reported as common causes of delay by other
investigators [26]. However, proportion of patients taking time to decide
to start therapy was higher in our cohort than previously described and
could be due to cultural differences or cost considerations, but requires
further study
Bronchoscopy, TTNA, and thoracentesis were most commonly
performed diagnostic procedures. Undergoing TTNA as the first
diagnostic procedure was associated with longer CT scan-to-treatment
time. This may reflect difficulty in obtaining timely slot for TTNA as
compared to bronchoscopy and was attributable to batch processing. TTNA
is done by limited number of radiologists and it is only done twice a week
at our centre whereas bronchoscopies are done daily. Batch processing is
known to cause waiting behind the date of processing and behaves like a
constraint in the flow of a process [30].Principles of lean thinking propose
efficient use of staff, resources, and technology to provide the highest level
of service and involve five steps to improve a selected process: value, the
value stream, flow, pull, and perfection. The goal of “flow” component of
these five steps is to eliminate the use of batching and queuing within
a process to ensure that a process is continuously worked on until it is
complete.
NSCLC patients experienced longer delay. Small cell carcinoma by
virtue of its aggressive nature on the one hand, and chemo-responsiveness
on the other is known to receive prompt treatment by creating a sense of
urgency. Similar shorter delay has been reported in small cell carcinoma
group by other investigators although they did not specify the reasons
[26].
The reason for correlation between male gender and longer CT scan-totreatment
interval could be due to more number of males and longer CT
scan-to-treatment interval in the patients treated with non-TKI therapy
versus more females (p=0.05) and shorter CT scan-to-treatment interval
(p=0.01) in TKI group. The shorter delay in TKI group can be attributed
to ease of initiation of oral therapy, lack of delay associated with staging
work-up, lung function tests, and resource intensive therapies such as
chemotherapy and radiotherapy.
Advanced age has been shown to be associated with less-timely care
[31]. Advanced age makes decision making difficult due to associated
co-morbidities and risk-benefit profile swaying more toward risks than
benefits. Such patients themselves often take longer time to decide if
they want to undergo therapy that entails side effects, and even when
they do, they often require relatively more preoperative tests, consults or
preparation for the operating room.
Mortality showed negative correlation with the timeliness, being
higher in the timely care group, and lower in the less timely group. This
was attributable to stage of the disease, reflecting patients with advanced
disease receiving prompt treatment. However, it also indicates that patient
with limited disease those who have the highest chance of better survival
if treated promptly paradoxically waited longer than those with advanced
disease in whom prompt treatment is unlikely to offer much benefit.
Surgically treated patients had a longer CT scan-to-treatment time than
those treated non-surgically mostly due to delay in “post-diagnosis”
period. This reflects the extra time needed to refer patients to thoracic
surgery units where additional treatment considerations are made like
staging scans. This raises the question about the efficiency of resource
allocation and reflects the area of weakness amenable to improvement.
Various approaches have been evaluated to improve timeliness of care
in lung cancer such as MDM, nurse-led care coordination, telemedicine
and a “two-stop” diagnostic process whereby patients receive CT, and
diagnostic procedure at the initial visit followed by formulation of a
treatment plan in a multidisciplinary meeting within 3 days [32-39] Out
of these the “two-stop” diagnostic process described by Laroche et al
and Murray et al has been shown to be significantly effective in reducing
diagnostic delay [35,38].
Our findings enable us to formulate the following recommendations
for timely care: 1) Since CT scan is more accurate than a chest radiograph,
the best and cost effective way to reduce miss-diagnosis of lung cancer as
TB will be to perform CT scan on all patients diagnosed as smear negative
TB, and having risk factors for lung carcinoma such as significant smoking
history in males, non-smoking Asian females, upper lobe involvement,
and self or family history of cancer. Upper lobe involvement alone should
not be considered as the hallmark of TB as commonly thought, because
the physiologic disparities in the perfusion-ventilation ratio, lymphatic
flow, metabolism, and mechanics, all of which result from the influence
of gravity across the various parts of the lung have been recognized as
important factors determining the upper lobe predominance of several
pulmonary diseases [40]. 2) Matching the first diagnostic procedure
closely to the radiographic features may help to reduce the number of
procedure needed and hence time taken to confirm the diagnosis. For
example, performing trans bronchial lung biopsy for bronchus sign,
endobronchial ultrasound guided transbronchial needle aspiration
for mediastinal lymph node and central masses. 3) In our institution
MDM is held every fortnightly, and on occasions, staging work-up is not
completed by the time of MDM. Conducting MDM on a weekly basis,
with special emphasis on stage IIIA cases along with attempt to complete
the diagnostic, staging, and operability work up where necessary prior to
the meeting so that the management decisions can be finalised during the
meeting. 4) Exploring the reasons why patients take a long time to decide
before embarking on therapy even after knowing they have lung cancer
also demands attention. Focus group discussions may help to unravel the
reasons for such delays.
In conclusion, longer delay in the management of early stage lung cancer
patients was seen indicating bias toward delivering expedious treatment to
symptomatic (by virtue of advanced stage) versus asymptomatic patients.
This implies that those with inherent chance of cure (early stage) had to wait
longer, with risk for progression of their disease. Undergoing CT guided
biopsy (transthoracic needle aspiration) to establish diagnosis, advanced
age, male gender, and NSCLC were the predictors of delay. Limited (twice
weekly) availability of CT guided biopsy (transthoracic needle aspiration),
failure of first diagnostic procedure to provide diagnosis, misdiagnosis of
lung cancer as TB, delayed patients` decision, and development of intercurrent
illness were the main causes of delay. Delay did not correlate with
poor survival due to greater proportion of early stage cancer in the delayed
group suggesting need for more efficient resource allocation.
Conflict of Interest Statement: A.V., A.C., A.L., D.Y.H.T.,
S.K.G., A.C.K., B.H., D.B.A.A., Y.W.L., and J.A. have no potential conflicts
of interest to report.
Summary at a Glance
We performed a retrospective study to elucidate predictors and causes of
delay in the management of lung cancer. CT guided biopsy (transthoracic
needle aspiration), advanced age, male gender, and NSCLC were the
predictors of delay. Limited (twice weekly) availability of CT guided
biopsy, misdiagnosis of lung cancer as TB, delayed patients` decision, and
inter-current illness were the main causes of delay.






