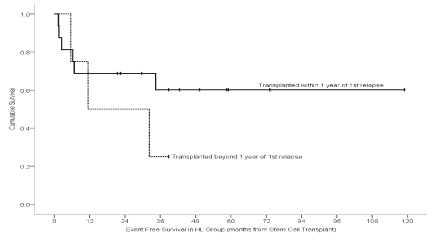
Figure 1: Event Free survival for HL sub-group by time to transplant.
Suleimman Al-Sweedan1* Ghaida Aljamal1 Khawar Siddiqui2 Mary Barria2 Rafat Jafri2 Amani Alkofedi2
1Department of Pediatrics, Jordan University of Science and Technology, Irbid, Jordan*Corresponding author: Suleimman Al-Sweedan, Department of Pediatrics, Jordan University of Science & Technology, Irbid, Jordan, Tel: 00962799051255; E-mail: sweedan@just.edu.jo
Background: Data on a cohort of lymphoma patients (Age ≤ 14 years) undergoing Stem Cell Transplantation (SCT) was analyzed to review the results of our treatment efforts.
Material and methods: A total of 39 patients with lymphoma underwent SCT at our institution from Jan 1993 to December 2016. Data on demographics, diagnosis, transplant-related parameters and outcome were abstracted from clinical databases and through medical charts review.
Results: Median age at transplant was 11.9 years (1-14.3). Twenty-one (53.8%) were with Hodgkin’s Lymphoma (HL) remaining 46.2% (18) were NonHodgkin’s Lymphoma (NHL). Median time to first relapse from diagnosis was 10.9 months (0.5-67.1) and time to transplant from the first relapse was 4 (1.4-35.5) months. Twenty-Nine (74.4%) were transplanted in autologous fashion (4 Burkitt’s, 3 Diffuse Large B-Cell, 2 anaplastic lymphoma, 20 HL) and 25.6% (10) allogeneic (5 Burkitt’s Lymphoma, 4 T-Cell and 1 HL Lymphocyte Predominant). Median time to ANC recovery in autologous group (n=29) was 11 days (9-14), while the same for the allogeneic group was 18 days (10-22) for 9 pts (1 early death) (P-Value: 0.022). aGVHD (+) was observed in two patients from the allogeneic group (1 skin Grade IV and Gut Grade II, 1 skin Grade 1). In autologous group in 65.5% (19) BEC (BCNU/ Cytoxan/VP-16) was the conditioning regimen while Cy/TBI (30%, 3) and Cy/VP-16/TBI (30%, 3) were used in allogeneic group. With a median followup of 58.5 (35.4-81.5) months, three-year Overall Survival (OS) was 0.300 ± 0.145 in Allogeneic Group as compared to 0.777 ± 0.081 in Autologous Group (P-Value: 0.001). Event Free Survival was 0.300 ± 0.145 in Allogeneic Group compared to 0.573 ± 0.094 in Autologous Group (P-Value: 0.012). 91.3% (21) of those who are alive are in remission (Autologous: 90%, Allogeneic: 100%)
Conclusion: In our cohort of patients, results of stem cell transplantation in terms of OS and EFS and transplant related toxicity are better in patients transplanted in autologous fashion.
Lymphoma; Stem cell transplantation; Outcome
Approximately 15% of all malignancies in children are attributed to Hodgkin and non-Hodgkin lymphoma. It is common to see cure rates of almost 100% for Hodgkin lymphoma (HL) and for nonHodgkin lymphoma (NHL) this figure is touching 85% [1]. During the last decades, the treatment of children with T-cell lymphoblastic lymphoma (T-LBL) and precursor B-cell lymphoblastic lymphoma (pB-LBL) has improved but despite that, patients with relapsed or refractory lymphomas still have a bad prognosis [2]. In HL even with an excellent prognosis in children, roughly 15% of the patient’s relapse [3]. Those patients who relapse at the early stage of treatment usually have a poor prognosis. Sensitivity to salvage therapy gives a chance of getting into those late relapse patients [4].
Chemotherapy still remains the backbone treatment for lymphoma, with different regimens depending upon the different type of lymphoma. High dose chemotherapy which is followed by stem cell transplant is usually considered a viable option for recurrent lymphoma by many oncologists [1]. There is a scarcity of data in relapse HL in children pertaining to the use of auto-HCT following the second remission [5]. For children with relapsed HL, HSCT has still considered an efficient and effective therapy resulting in longterm cures [6]. It has also been demonstrated as a salvage therapy in approximately half of the HL pediatric patients failing the first line therapy with chemosensitivity to the second line therapy [7].
Myeloablative conditioning (MAC) with Auto-HCT followed by reduced intensity conditioning (RIC) with allogeneic hematopoietic cell transplantation (Allo-HCT) in children with relapsed or refractory lymphoma has successfully been tested as safe and effective with 10 years EFS of 59.8% in HL and 70% in NHL [8]. For highly aggressive Non-Hodgkin lymphoma (NHL) such as newly diagnosed Primary Central Nervous System Lymphoma, a high-dose of chemotherapy with BCNU (or busulfan) and thiotepa followed by HCT-ASCT is advantageous and more efficient but remain questionable if the approach is superior as compared to conventional chemotherapy [9]. In primary refractory or relapsed aggressive NHL, mantle cell lymphoma and relapsed follicular or peripheral T-cell lymphoma have better survival with autologous hematopoietic stem cell transplantation (SCT) which may improve progression-free survival as a consolidation treatment after first completion or partial remission [10]. It is relevant to know the patient’s risk of relapse before the start of treatment in other to figure out whether it’s beneficial or not. There are certain disease-related factors to determine the low and high-risk relapse like the International Prognostic Factor Index system and The Tumor Score System and, treatment-related factors such as the dose of chemotherapy and rapidity of response. Patients who have not responded to initial therapy might have a very poor outcome to standard relapse treatment. Platinum-based, mitoxantrone-based and ifosfamide-based chemotherapy drugs are three groups of drugs being used as post-relapse therapy for aggressive lymphoma [11].
In order to evaluate the treatment efforts in our institution, we conducted this retrospective review on 38 pediatric patients who underwent hematopoietic stem cell transplant (age ≤ 14 years) with relapsed or refractory lymphoma between 2006 and 2015.
A retrospective review of the medical record of pediatric patients (age at diagnosis ≤ 14 years) with relapsed or refractory lymphoma as the primary indication for stem cell transplantation (SCT) was carried out after getting approval from the Institutional Review Board (IRB) of King Faisal Specialist Hospital and Research Centre (KFSHRC&RC), Riyadh, Saudi Arabia. Data on demographics, diagnosis, transplantrelated parameters and the outcome for thirty-eight (38) patients transplanted from January 2006 to December 2015, was abstracted from clinical databases and through medical charts review for this retrospective cohort study. The dataset was processed and analyzed using IBM-SPSS Version 20 (IBM Corporation, 1 New Orchard Road Armonk, New York 10504-1722, United States).
Patients’ and disease characteristics along with transplant related parameters on 52.6% (20) with Hodgkin’s Lymphoma (HL) and remaining 47.4% (18) Non-Hodgkin’s Lymphoma (NHL) are listed in Table 1. GVHD prophylaxis was administered in nine (50%) of NHL patients - one had CSA only, two (2) got CSA with steroids and the remaining received CSA with MTX. All our patients received chemotherapy for conditioning (Table 1). Nine (9) from the NHL group underwent Total Body Irradiation (TBI). For our patient with Allogeneic SCT, the GVHD prophylaxis commonly administered were Cyclophosphorine (CSA), Methotrexate (MTX) with or without steroid and bone marrow was the most common HSCT source. In the autologous group, BEC (BCNU/Cytoxan/Vp16) was the common chemotherapy conditioning regimen, while for the allogeneic group, CY/TBI and CY/VP16/TBI were commonly used for conditioning regimen with or without TBI.
| Hodgkin’s Lymphoma | Non-Hodgkin’s Lymphoma | Total | |
| 20(52.6) | 18 (47.4) | 38 | |
| Gender | |||
| Female | 5 (25) | 8 (44.4) | 13 (34.2) |
| Male | 15 (75) | 10 (55.6) | 25 (65.8) |
| Age | |||
| At diagnosis, Median (Min-Max) | 10.5(4.5-13.2) | 6.0(0.6-13.9) | 9.0(0.6-13.9) |
| At SCT, Median (Min-Max) | 12.6 (6.5-14.8) | 7.2 (1.0-14.3) | 11.5 (1.0-14.8) |
| Graft type-Allogeneic transplant (n=9) | |||
| Burkitt lymphoma | None | 5 | 5 |
| Precursor T-cell lymphoblastic lymphoma | None | 1 | 1 |
| T-cell lymphoma, NOS | None | 3 | 3 |
| Stem cell source-Bone marrow | None | 6 | 6 |
| Stem cell source-Cord Blood | None | 3 | 3 |
| Graft type - Autologous transplant (n=29) | |||
| Bukrkitt lymphoma | None | 4 | 4 |
| Hodgkin lymphoma, lymphocyte-rich | 1 | None | 1 |
| Hodgkin lymphoma, nodular sclerosis | 16 | None | 16 |
| Hodgkin lymphoma, mixed cellularity | 3 | None | 3 |
| Diffuse large B-cell lymphoma | None | 3 | 3 |
| NK/T cell lymphoma, nasal and nasal-type | None | 1 | 1 |
| Precursor T-cell lymphoblastic lymphoma | None | 1 | 1 |
| Stem cell source-Bone marrow | 2 | 2 | 4 |
| Stem cell source-Peripheral blood | 18 | 7 | 25 |
| Time to 1st Relapse from Diagnosis (months), Median (Min-Max) | 11 (0.5-67.1) | 7.8 (1.7-32.5) | 10.9 (0.5-67.1) |
| Time to SCT from 1st Relapse (months), Median (Min-Max) | 4.8 (2.3-35.5) | 4.1 (1.4-7.7) | 4.2 (1.4-35.5) |
| Disease status at SCT | |||
| CR-2 | 15 (75) | 18 (100) | 33 (86.8) |
| CR-3 | 5 (25) | None | 5 (13.2) |
| HLA type | |||
| HLA identical siblings (Bone marrow) | None | 6 | 6 |
| Unrelated, 1-AG mismatch (Cord blood) | None | 2 | 2 |
| Unrelated, 2-AG mismatch (Cord blood) | None | 1 | 1 |
| Conditioning Regimen (chemotherapy) | |||
| BEC | 14 | 5 | 19 |
| CEC | 1 | 1 | 2 |
| CEM | 1 | None | 1 |
| Cy/BCNU/VP-16 | 4 | 2 | 6 |
| Cy/VP-16 | None | 1 | 1 |
| Cy/TBI | None | 3 | 3 |
| Cy/ATG/TBI | None | 2 | 2 |
| Cy/VP-16/TBI | None | 4 | 4 |
| Outcome parameters | |||
| Death | 4 (25.0) | 12 (66.7) | 16 (42.1) |
| Alive in CR | 11 of 16 | 6 of 6 | 17 of 22 |
| Values are in n (%) for discrete data. |
Table 1: Patients, disease characteristics and transplant related parameters (n=38).
Absolute Neutrophil Count (ANC) recovery occurred in all but one NHL case transplanted in allogeneic fashion, which had an early death. Median time to ANC recovery in HL cases was 11 days (Min: 9, Max: 19) while median time to ANC recovery for NHL group was 14 days (Min: 10, Max: 22). Median platelets recovery time for HL patients was 21 days in contrast to 25 days for NHL cases. Evaluation of engraftment near D100 revealed two counts of graft failures, both in allogeneic transplantation group with NHL as the primary indication for transplant, one of them was transplant related mortality (TRM). Acute GVHD was observed in our NHL patients with an incidence of 5.1%. One patient had Grade 1, skin GVHD while the other had Grade 4 skin along with Grade II in the gut; the latter then succumbed to this event. Stem cells source for both of these patients were bone marrow from HLA identical siblings (female) with CSA and MTX as GVHD prophylaxis regimen. The rate of fungal infection within Day 100 of stem cells infusion in NHL was 11.1% (2). For both cases, the infection contributed towards their expiry; both were related to Aspergillus. Three of our NHL cases contracted viral infection (16.7%), 2 of them were CMV and one was herpes zoster. Bacterial infections were seen in 23.8% (5) in HL and 38.9% (7) in NHL cases. Cumulative incidence of any infection during the first one hundred days of SCT infusion was 25% (5) in HL and 50% (9) in NHL patients. Veno occlusive disease was observed in only one NHL case. Episodes of encephalopathy, interstitial pneumonia or hemorrhagic cystitis were not observed during the early 100 days of transplant. Grade 3 and above mucositis was seen in 3 cases, 1 with HL and the remaining cases with NHL. Secondary malignancy was seen in two NHL patients; one was diagnosed with A.M.L and the other had a papillary glioneuronal tumor of the brain. Mortality rate was 20% (4) in our HL group patients, while the same was 66.7% (12) in NHL. Post-SCT relapse of primary disease was seen in 35% (7) in HL and 38.9% (7) in NHL. With a median follow-up time of 49.3 months (95% CI: 19.1-79.5), the five years probability of overall survival of our cohort of patients for HL was (0.680 ± 0.149) and (0.444 ± 0.117) for NHL. Observing six (15.4%) deaths, 14 (35.9%) post-SCT relapses and 1 (2.6%) new malignancy (came first) as events, the probability of five years eventfree survival (EFS) was (0.520 ± 0.118) for HL patients and was (0.370 ± 0.119) for NHL patients. EFS for HL patients transplanted within one year of relapse (n=16, Fig 1) was not statistically significantly different from those who underwent SCT beyond one-year post-relapse (0.602 ± 0.129 vs.0.250 ± 0.217, P-Value: 0.496). All the patients with NHL as the primary indication of SCT were transplanted within one year of the first relapse. Among the NHL group, the probability of five-year overall survival was (0.222 ± 0.139) in allogeneic compared to (0.667 ± 0.157) in autologous transplants, with a median follow-up of 60.3 months (P-Value: 0.035). The probability of five-year EFS was better in autologous (0.533 ± 0.173) than allogeneic transplanted sub-group (0.222 ± 0.139, P-Value: 0.035, (Figure 1,2). In HL group 68.8% (11 of 16) and, 100% (6 of 6) in the NHL group were alive in CR.

Figure 1: Event Free survival for HL sub-group by time to transplant.
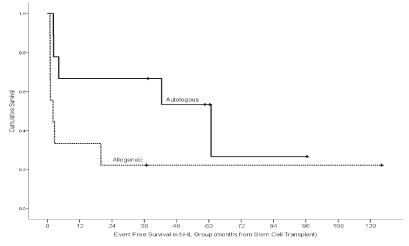
Figure 2: Event Free survival for NHL sub-group by graft type.
For early Hodgkin Lymphoma (HL) relapse patient, Auto HSCT following high-dose chemotherapy in pediatric patients has been reported to be an effective salvage therapy. However, the evidence for its superiority to conventional therapy in terms of randomized clinical trials is lacking [2,3]. Data using autologous or allogeneic hematopoietic stem cell transplant (SCT) as an effective therapy for refractory NHL with pediatric population is still limited [12]. In recent years there have been encouraging reports on HSCT as a salvage therapy for pediatric patients with lymphoma who have failed the first line therapy but showed chemo-sensitivity to the second line treatment [7]. In this study, we reported data on a small group of 38 children with lymphoma who underwent HSCT at our institution using established CIBMTR protocols.
Our finding shows that the post-transplant hematopoietic recovery was comparable with other groups in terms of median time [13,14]. These data correlated with findings in a group of pediatric patients with allogeneic PBSCT vs allogeneic BMT, the median time to achieve Absolute Neutrophil Count (ANC) was 11 and 15 days, platelet engraftment was 12 and 21 days [15]. Fukano, et al. recently reported some number of patients for autologous vs. allogeneic HSCT with Anaplastic large cell lymphoma (ACLC), Treatment related mortality was 12% and 25% [16]. On the other hand, a study with 10 NHL patients underwent autologous peripheral blood stem cell transplantation (APBSCT) with a conditioning regimen of BEAM or BuCy, TRM was not detected [17]. For patients with relapsed HL who underwent auto and allogeneic SCT with 100- day TRM was under 10% [18]. Which was almost similar to our result as Transplant-related mortality (TRM) was 21.1% (8 of 38) -5% (1 of 20) in HL patients and 38.9% (7 of 18) in NHL patients.
An incidence of 5.1% of acute graft-versus-host (aGVHD) was observed in our group of patients, grade I Skin GVHD n=1 and grade IV Skin GVHD with grade II Gut GVHD n=1using CSA and MTX as GVHD prophylaxis regimen. Miyagaki S, et al. Reported an 11- year- old patient with relapsed ALCL who underwent allo-HSCT with reduced intensity conditioning (fludarabine, melphalan, and low-dose TBI), developed Grade II aGVHD treated successfully with methylprednisolone. No chronic GVHD was encountered [19]. Gross, et al. in an NHL with allogeneic SCT, the incidence of aGVHD at day 100 was 43% with grade II-IV. Chronic GVHD (cGVHD) in 5 year incidence was 16% [20].
In St. Jude experience, early infections (bacterial, fungal, viral, or parasitic) occurred post 0-30 days auto-SCT in 66 (21%) of solid tumor or lymphoma which is not associated with mortality [21]. Fungal infection was observed in our small group of patients within 100 days of stem cell infusion with 11.1% of NHL, n=2. Our study of 5 patients with 23.8% in HL had the complication of bacterial infection and NHL in 7 patients with 38.9%. Hussein, et al. from Amman, Jordan reported that out of 65 patients identified with lymphoma and other malignant cases, out of these patients 33 were documented with a bacterial infection, which is 50% during the first 100 days after AHSCT in children [22]. Viral Infection was observed in our group with 3 NHL patients and a rate of 16.7% Similar report from Japanese pediatric leukemia/ lymphoma study group (JPLSG) B-NHL03 protocol with4 toxic deaths associated with viral infection occurring in NHL which assessed small group of patients after allogeneic SCT following on rituximab combined salvage therapy [23].
In our cohort study, 2 patients developed secondary neoplasm malignancy in NHL patients and these are Acute Myeloid Leukemia and Papillary Glioneuronal tumor of the brain. An author described by Ogami A, et al. in an 8-year-old girl NHL developed Acute Promyelocytic Leukemia t-APL after administering chemotherapy, the development appears to be uncommon in children with NHL [24]. Another study of which a 10-year cumulative incidence of 7.9% was observed in NHL with a second malignancy treated with HDT-ASCT between 1987 and 2008 (n-578) [25]. Mortality rate of 20% for 4 HL and 66.7% for 12 NHL patients. In Post-SCT relapse, 35% for 7 HL and 38.9% for 7 NHL. Hazar, et al. the probabilities of the overall survival at five years was 63.1% and the EFS was 54.3% in an evaluation of 66 patients rrHL relapsed or refractory Hodgkin lymphoma who underwent Auto HSCT [26]. A report from the Turkish bone marrow transplant registry shows that the overall survival (OS) was 65% and event-free survival (EFS) rates were 48%, an outcome of 62 relapsed or refractory non-Hodgkin lymphoma (NHL) who underwent hematopoietic stem cell transplantation (HSCT) [27]. In our experience, the five-year probability for overall survival for HL and NHL were (0.680 ± 0.149) and (0.444 ± 0.117) respectively. Event-free survival for HL and NHL were (0.520 ± 0.118) and (0.370 ± 0.119). In our cohort of patients, results of stem cell transplantation in terms of OS and EFS and transplant related toxicity are better in patients transplanted in autologous fashion.
The authors would like to acknowledge the efforts of the staff of the Central Data Unit, Department of Pediatric Hematology/Oncology in data collection, its quality assurance, analysis and results reporting.
The study was approved by the Institutional Review Board (IRB) of KFSH&RC via Approval Number 2141033. Waiver of informed consents/assents was obtained from the IRB for being a retrospective non-interventional study.
Download Provisional PDF Here
Article Type: RESEARCH ARTICLE
Citation: Al-Sweedan S, Aljamal G, Siddiqui K, Barria M, Jafri R, et al. (2018) Stem Cell Transplantation (SCT) in Pediatric (Age ≤ 14 Years) Relapsed or Refractory Lymphoma Patients a Result from Single Institution. J Blood Disord Med 3(1): dx.doi.org/10.16966/2471-5026.122
Copyright:© 2018 Al-Sweedan S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access