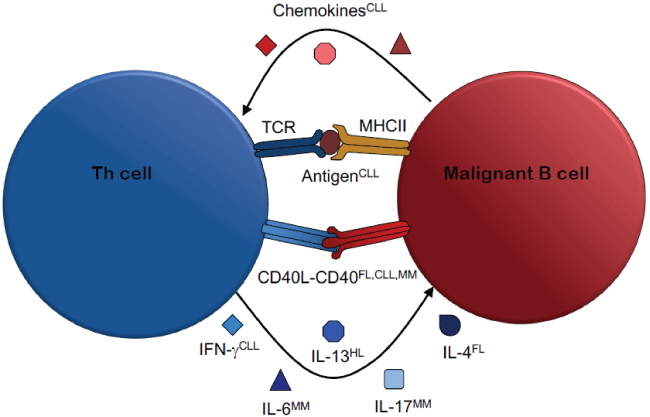
Figure 1: Mechanisms of Malignant Th cell-B cell Interactions
Simone Burgler*
Experimental Infectious Diseases and Cancer Research, University Children’s Hospital Zurich, Zurich, Switzerland*Corresponding author: Simone Burgler, Experimental Infectious Diseases and Cancer Research, University Children’s Hospital Zurich, Zurich, Switzerland, E-mail: simone.buergler@kispi.uzh.ch
Collaboration of T helper cells and B cells is central for the generation of high affinity antibodies with distinct effectors functions, and thus for the establishment of effective immune responses. Physiological T cell help for B cells takes place in germinal centers in peripheral lymphoid organs, where follicular T helper cells interact with mature, antigen-stimulated B cells. Occasionally, B cells undergo malignant transformation, which may lead to the development of leukemia or lymphoma. In nearly all cancers, the tumor cells critically depend on interactions with the tumor microenvironment for growth and survival. Since many B cell malignancies develop in germinal centers - the place of physiological T helper cell-B cell interaction - T helper cells represent a main component of the tumor microenvironment of B cell leukemia and lymphoma. Thus, while crucial for the development of an effective immune response, the interaction between T helper cells and B cells on the flip side contributes the development and pathogenesis of B cell malignancies. This mini-review discusses the mechanisms underlying T helper cell-mediated support of malignant B cells in leukemia and lymphoma. Given the importance of the tumor microenvironment in cancer pathogenesis, targeting these malignant interactions may increase treatment efficiency and reduce disease relapse.
T Helper Cells; B cells; Leukemia; Lymphoma; B cell malignancies; T helper cell-B cell interaction; Tumor microenvironment
CLL: Chronic Lymphocytic Leukemia; FL: Follicular Lymphoma; HL: Hodgkin Lymphoma; MHC: Major Histocompatibility Complex; MM: Multiple Lymphoma; TCR: T Cell Receptor
Antibodies are a central arm of the adaptive immune system. Highly diverse and equipped with diverse effectors functions, antibodies recognize and neutralize invading pathogens by various mechanisms. While B cells are the producers of antibodies, they depend on help from T helper (Th) cells for the generation of high affinity antibodies with distinct effectors properties. Thus, the establishment of a specific and efficient immune response requires a close collaboration of Th cells and B cells.
Th cells are generated in the bone marrow (BM) but mature in the thymus. Naïve Th cells leave the thymus and migrate to the periphery, where they may encounter antigenic peptides presented by antigen-presenting cells. Upon stimulation, Th cells proliferate and differentiate into one of several effector subsets that are distinct in phenotype and function. Best characterized among these are the pro-inflammatory Th1 cells, expressing interferon (IFN)-γ, and the Th2 cells, producing interleukin (IL)-4, IL-5 and IL-13 [1].Besides further effector lineages such as Th17, Th9 or Th22, several Th cell subsets with regulatory or suppressive functions - called regulatory T (Treg) cells-exist [2]. In addition, follicular helper T (Tfh) cells make up a unique population of Th cells distinct from extrafollicular and peripheral Th cells [3].
B cells develop and mature in the BM and subsequently migrate to the secondary lymphoid organs for the antigen-dependent phase of their development. While this process can be independent of T cell help, B cells conventionally engage in T cell-dependent responses and receive stimulation by CD40L, IL-4 and IL-21 from Tfh cells [4]. B cells further develop either into short-lived plasma cells, or into GC B cells that give rise to long-lived memory B cells and plasma cells. Importantly, the interaction with Tfh cells leads to the upregulation of activation-induced cytidine deaminase (AID), a DNA-editing enzyme and that initiates somatic hypermutation (SHM) and class switch recombination (CSR), the basic mechanisms creating high affinity antibodies with diverse effector functions [5].
During their development, B cells may undergo malignant transformation, resulting in leukemia or lymphoma. Such transformations are frequently initiated by genetic events leading to aberrantly expressed proteins that promote growth and survival of the cells. The mutations, however, are usually not sufficient for cancer development. Instead, malignant B cells critically depend on interactions with cells of their microenvironment in order to survive and expand [6-8].
B cell malignancies often arise from GC B cells. Thus, the cells within GC represent key collaboration partners of malignant cells during pathogenesis, progression and relapse of leukemia and lymphoma. Besides non-hematopoetic cells such as mesenchymal stromal cells and fibroblasts, the GC harbors Tfh cells that support B cells in their physiological maturation and function. Interestingly, malignantly transformed B cells seem to retain their ability to interact with Th cells, and are therefore still capable of profiting from Th cell help. Thus, the same Th cell-mediated support that is crucial for an adaptive immune response can-when directed towards malignant B cells-promote lymphoma or leukemia (Figure 1).

Figure 1: Mechanisms of Malignant Th cell-B cell Interactions
Follicular lymphoma (FL) is an indolent lymphoma arising from GC B cells. Both non-hematopoietic cells as well as Th cells play a crucial role in supporting FL cell growth and survival [9]. Tfh cells from FL-affected lymph nodes express increased levels of IL-2, IL-4, IFN-γ and TNF [10] and seem to support FL cells by IL-4 [11,12]. Besides cytokines, also ligation of CD40 with CD40L plays a role. FL cells showed an increased survival when stimulated by CD40 cross linking in vitro [13] as well as upon cognate interaction with Th cells [14], and it has been suggested that CD40L stimulation protects FL cells from TRAIL-mediated apoptosis in a NF-κB-dependent manner [15].
Burkitt’s lymphoma (BL) is an aggressive B cell cancer, probably arising from GC B cells [16] BL is strongly associated with the EpsteinBarr virus (EBV), even though the pathogenic mechanism is not clear [17,18]. The role of Th cells in BL development and progression is highly controversial. Several studies showed that EBV-specific Th cells could kill or limit proliferation of BL cell lines or EBV-transformed B cells [19-27]. Others, in contrast, have reported that EBV-specific Th cells induced B cell proliferation [28], and in several mouse models such EBV-specific Th cells were even required for lymphomagenesis [29-31]. Two studies found both a killing and supportive role for Th cells [32,33], suggesting that the function of Th cells in BL and other EBV-associated malignancies is likely to be context-dependent.
In Hodgkin lymphoma (HL), infiltration of certain Th cell subsets is correlated with reduced overall patient survival [34,35]. Several cytokines seem to have a stimulatory effect on malignant cells in HL, one of which is the Th2 cytokine IL-13 [36]. Nevertheless, the source of this cytokines is still unclear. Thus, a direct role of Th cells in HL development or expansion remains to be demonstrated.
Chronic lymphocytic leukemia (CLL) is a malignancy of mature clonal B cells, although the precise cell of origin is still under debate [37]. CLL cells proliferate in pseudofollicles in secondary lymphoid organs and in the BM, where they receive support from cells of the stromal microenvironment [38]. Th cells are actively recruited by CLL cells via chemokines to infiltrate such CLL pseudofollicles [39,40]. Recently, we found that these Th cells recognized antigen derived from autologous CLL cells and stimulated CLL cell activation and proliferation in an antigenand CD40L-dependent manner in vitro and in an in vivo xenograft model [41]. Interestingly, the patients-derived CLL-specific Th cells had a Th1- like phenotype, characterized by high IFN-γ secretion. IFN-γ upregulated CD38, a marker of poor prognosis in CLL in a mechanism involving IFN-γ-induced binding of the transcription factor T-bet to two consensus sites in 5 regulatory regions of the CD38 gene [42,43]. Consistently, T-bet expression in peripheral blood CLL cells significantly correlated with CD38 expression. Thus, it seems that Th cell promote the development of a more aggressive CLL subset through secretion of IFN-γ.
CLL cells express polyreactive and/or autoreactive BCR that provide a certain level of constant signaling [44,45]. However, sustained BCR signaling can induce anergy and apoptosis. In fact, CLL cells are considered to be autoreactive B cells that may be rescued from anergy by stimuli from the microenvironment [46,47]. Consistently, we found that stimulation by CD40L activated the kinase Syk in CLL cells, a component that is shared by the BCR and the CD40 signaling cascade. This suggests that Th cells contribute to CLL development by rescuing CLL cells from anergy through CD40L stimulation [48].
Multiple myeloma (MM) is a malignancy characterized by the expansion of plasma cell-derived myeloma cells in the BM. The BM of MM patients displayed increased numbers of T cells [49], and CD40 stimulation induced MM cell migration, which is associated with MM disease progression [50]. CD40 stimulation also triggered secretion of IL-6 by MM cells, which may mediate MM cell proliferation in an autocrine and/or paracrine mechanism [51]. In addition to CD40Lmediated stimulation, MM-specific Th cells could also support autologous MM cells by secreting cytokines [52]. Very recently, we demonstrated that polyclonally activated allogeneic as well as autologous Th cells stimulated blastogenesis and proliferation of MM cells in a CD40L-dependent manner [53]. Together with the previous reports by others, this suggests that CD40L stimulations is a key mechanism in Th cell-mediated MM cell support, but cytokines like IL-6 and IL-17 are important components as well.
The tumor microenvironment plays a key role in supporting malignant cells. In B cell leukemia and lymphoma, the malignant B cells seem to have retained the ability to receive help from their physiological interaction partners, the Th cells. Consistently, a cancer-supportive role for Th cells has been described in various types of B cell malignancies, although the detailed mechanisms remain to be determined. Effective anti-cancer therapies should involve targeting the cells of the tumor microenvironment. Thus, research efforts leading to the identification and characterization of tumor-promoting collaboration between Th cells and malignant B cells may provide novel strategies for therapies aiming to target the tumor microenvironment.
Download Provisional PDF Here
Article Type: Review Article
Citation: Burgler S (2017) Malignant Interaction between B Cells and T Helper Cells. J Blood Disord Med 2(1): doi http://dx.doi.org/10.16966/2471-5026.115
Copyright: © 2017 Burgler S. This is an openaccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access