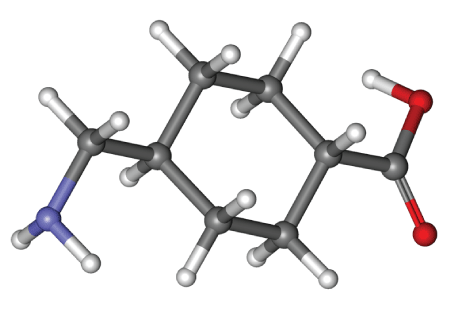
Figure 1: Molecular structure of tranexamic acid, a synthetic lysine derivative.
John R Tuttle*
Department of Orthopedic Surgery, Warren Alpert Medical School of Brown University, USA*Corresponding author: John R Tuttle, Department of Orthopedic Surgery, Warren Alpert Medical School of Brown University, USA, Tel: +1 408-816-2779; Fax: +1 408-837-0557; E-mail: tuttle.78@gmail.com
Tranexamic acid (TXA) has been in use world-wide for over 50 years to help reduce blood loss and transfusion rates. TXA is an antifibrinolytic that has found a growing interest in multiple surgical fields, especially in Orthopedics over the past decade. As the evidence and applications continue to expand, it is important to understand the role this drug is playing in the care of patients. The purpose of this publication is to briefly summarize the history of TXA, its development, its current applications, and future directions for exploration.
Tranexamic acid; TXA; Antifibrinolytic; Transfusion; Blood-loss; Review; History
Hemorrhage and exsanguination have continued to stymie physicians and surgeons despite all of the advancements of scientific medicine. Transfusion remains a mainstay of treatment; however cost and risk persist and limit our willingness to be satisfied with this tool alone. Tranexamic acid (trans-4-[aminomethyl] cyclohexane carboxylic acid) figure 1 or TXA is a synthetic lysine derivative that was developed in Japan by Shosuke and his wife Utako Okamoto in 1962. It is an antifibrinolytic that competitively inhibits lysine-binding sites on plasminogen molecules. This prevents the activation of plasmin and thus preserves the function of fibrin in clot formation. At higher concentrations, TXA also noncompetitively inhibits the binding of plasmin to fibrin, further preserving formed clot. Lysine by the same mechanism displays antifibrinolytic properties, which was the reason it provided a reasonable synthetic platform. E-aminocaproic acid was originally created by modifying lysine, leading to a nearly 10- fold increase in antifibrinolytic activity. This drug was marketed worldwide for its ability to reduce bleeding. In comparison, TXA is nearly ten times more effective than E-aminocaproic acid. Bleeding is thereby reduced by shifting the clotting/fibrinolysis balance in favor of clotting by a much more potent molecule [1]. Synovial fluid is a transudate of serum and small molecules typically diffuse across the synovial membrane unimpeded. TXA similarly crosses from the bloodstream into synovial fluid and reaches equilibrium with serum concentrations when administered intravenously [2]. It undergoes glomerular filtration via the kidneys without chemical modification, active excretion or absorption. Nearly 95% of the dose is excreted through the urine by 48 hours. The IV half-life is between 2-3 hours and the oral bioavailability is 30-50% [3]. Since its creation, TXA has proven useful in neurosurgery, urologic surgery, obstetrics and gynecology, trauma and trauma surgery, as well as orthopedic surgery [4]. The effectiveness of TXA in reducing bleeding in surgical and trauma patients has been repeatedly demonstrated [5,6,7].

Figure 1: Molecular structure of tranexamic acid, a synthetic lysine derivative.
The safety profile of TXA is very good, there are no reports of allergy or anaphylaxis, however its use may carry risk [2]. There is evidence in the trauma population that giving TXA over three hours after the onset of significant hemorrhage may contribute to increased mortality [8,9]. In addition, multiple reports have surfaced of intrathecal TXA leading to the production of seizures [10,11]. It is proposed that TXA exerts a GABA receptor antagonistic effect and thereby in higher concentrations may lead to seizure [12].
Thromboembolic events continue to be a concern as well. A review of the literature performed by Chan et al. [13]. found no conclusive evidence that TXA contributes to thromboembolic events. Instead they speculate that patients’ pre-existing thrombophilia conditions combined with procoagulant factors are the underlying cause of thromboembolic events in patients that receive TXA. They conclude however that patients with known thrombophilia should be approached with caution when considering TXA administration. The known risks of TXA encourage scrutiny in its application, which continues to expand.
TXA use has found renewed and new interest in multiple fields, including cardiac surgery, spine surgery, obstetrics and gynecology, and orthopedics [14-17]. The indications and data supporting them are rapidly increasing. Orthopedics especially has seen an explosion in interest in this drug over the past decade. Evidence continues to mount for its routine application in the field.
The current use of TXA in orthopedic surgery lies largely in spine surgery, total joint arthroplasty and trauma. There may be applications in other sub-specialties, however this remains undetermined.
Spinal surgery may lead to excessive blood loss, especially in large deformity correction and multi-level fusions. Multiple clinical trials have demonstrated a consistent reduction in blood loss and in some instances, significant transfusion reduction [18,19]. Postoperative complications remained unaffected by TXA administration in these trials. Despite its widespread use in spine surgery, there is no consensus regarding the dose and timing of TXA administration [20].
Total joint arthroplasty literature has erupted with studies providing ample evidence for TXA in arthroplasty surgery. Research continues to attempt to pin down the best way to implement it. TXA has been administered via oral, intravenous, and topical routes. It is unclear which method of administration is most efficacious and safe [21]. While concern may persist regarding IV administration of TXA, large-scale clinical trials have not found any increase in adverse events [22,23]. Routine use in arthroplasty surgery has proven to be cost-effective as well by reducing transfusion rate [24,25]. All patients receiving a primary total hip or knee stand to benefit from TXA regardless of sex, age, BMI, or preoperative hemoglobin level [26,27]. Both IV and topical applications are effective in reducing blood loss and transfusions in this population [28,29]. At this time, it is clear that TXA should be routinely used during arthroplasty surgery. What remains unknown is the best protocol to adopt. A recent study demonstrated that oral TXA may be just as effective as IV TXA in total joint arthrosplasty transfusion reduction. With oral TXA being significantly cheaper than IV dosing, this may lead to a shift in route administration in the future.
With its success in total joint arthroplasty, the extension of TXA use into hip fracture surgery, especially hemiarthroplasty (partial hip replacement) was inevitable. Multiple studies have found a reduction in blood loss and transfusion rates when TXA was used [30,31]. This is of particular interest in this patient population as they are more susceptible to anemia and its adverse effects. In addition, these patients lose a significant amount of blood prior to surgery and thus are more likely to require transfusion compared to those undergoing elective surgery [32]. Another consideration in these patients is the fact that they retain native cartilage in their hip. Recent in vitro data indicate that TXA can damage cartilage and is chondrotoxic at concentrations ≥ 50 mg/ml [33]. Further data is required to draw clinically relevant conclusions regarding the effect of TXA on cartilage.
Tranexamic acid has proven it belongs in the physician’s armamentarium to reduce blood loss and prevent transfusions. While a great deal is known about TXA and its benefits, much remains to be discovered regarding the best practice for its implementation and the full breadth of its application.
The author has no disclosures, no conflicts of interest regarding this publication.
Download Provisional PDF Here
Article Type: Mini Review
Citation: Tuttle JR (2016) Tranexamic Acid – A Brief Review and Update. J Blood Disord Med 1(3): doi http://dx.doi.org/10.16966/2471-5026.109
Copyright: © 2016 Tuttle JR. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access