Abstract
The MTT (3-(4,5-dimethylthiazol-2-yl) 2,5-diphenyl tetrazolium bromide) assay is able to discriminate living cells; the signal generated is dependent
on the degree of activation of the cells. In this paper, we show how the tetrazolium dye procedure conducted on a limited number of cells (1x103
)
is enhanced under basic condition. Moreover, the basic condition generate a more stable dye where only one peak is highlighted at 545 nm,
abolishingbackgrounds. A solution of NaOH 7N was usedas formazan enhancer. The basic milieu turns the formazan dye deriving form low
number of cells from brown to intense purple and provides a stable and more sensitive way, leading to high effectiveness of the assay. This
strategy can be a useful tool for spectrophotometer analysis where the limit of detection of formazan dye is a problem and a plate reader is not
available.
Keywords
Tetrazolium dye; MTT; Sodium Hydroxide
Introduction
The MTT (3-(4,5-dimethylthiazol-2-yl) 2,5-diphenyl tetrazolium
bromide) assay, originally described by Mosmann, has been used to
develop a quantitative colorimetric assay for mammalian cell survival
and proliferation and the method was successively modified for better
resolution [1-3]. For survival and proliferation determination, other
methods, such as [3
H]-thymidine uptake (3
H-TdR) or trypan blue are
available but actually, the MTT assay replaced both. The signal generated
is dependent by the capability of a mitochondrial dehydrogenase of
living cells, when cytochrome c is not released, to produce formazan
from tetrazolium salt [4,5]. However, the original technique has several
technical limitations, namely a less than optimal sensitivity, a variable
background due to protein precipitation on adding an organic solvent
to dissolve the blue formazan product, and a low solubility of the
product. These problems have been overcome by several modifications:
i) avoidance of serum and phenol red in the incubation medium; ii) use
higher concentration of MTT, iii) elimination of the medium containing
MTT after the reaction and subsequent use of pure propanol, isopropanol
or ethanol to rapidly solubilize the formazan crystals and iv) use of a more
judicious reference wavelength in a dual wavelength spectrophotometer
[3]. Due to these modifications the reliability and sensitivity of the test have
been increased to the point where it can replace the [3
H] thymidine uptake
assay, in many cases, to measure cell proliferation or survival in growth
factor or cytotoxicity assays. The results can be read on a micro-plate
reader or in a standard spectrophotometer. It is worth to note that MTT
assay is very easy and main advantages are: i) elimination of radioactive
compounds; ii) reproducibility; iii) performance and quantification facility
and iv) rapidity. However, there is a further technical limitation, due to
the variability among read peaks [5-8]. Here, we introduced a further
modification to the tetrazolium dye technique, using basic condition
(NaOH 7 N) that yields an improved stability and sensitivity of the dye. To
verify the stability (after reduction by cells in formazan) the absorbance
was monitored in a range of wavelength between 400 and 700 nm. Briefly,
MTT was dissolved in a stock solution: 5 mg/ml MTT (Sigma) in RPMI-
1640, DMEM or DMEM/F-12 without phenol red and serum (Gibco,
Invitrogen). This solution was filtered through a 0.2 µm filter and stored at
4°C. For the assay 1:40 dilution of the 5 mg/ml stock was used in a volume
of 1.0 ml for each well of 24-wells plate. 1 × 103
cells were incubated at
37°C for 120 minutes. As a first step, the converted dye is solubilized with
1 ml acidic isopropanol/0.04 N HCl, pipetted up and down several times
to assure that the converted dye dissolves completely and then transferred
into a 1.5 ml eppendorf tube and centrifuge at 13,000 rpm for 2 minutes.
The supernatant is transferred into a new eppendorf tube and 25 µl
Sodium Hydroxide (NaOH) 7 N were added to the dye solution, vortexed
for 10 secs and centrifuged at 13,000 rpm for 2 minutes. Conversely, to the
method found for determination of protein concentration, namely WST-
8 formazan (Dojindo), in which MTT is reduced to formazan by basic
condition, our method is based on the capability of the already reduced
formazan to produce, under basic condition, higher intensity color that
become more stable when monitoring the absorbance [9]. We observed
that the formazan dye is brown at acidic pH, and turns to intense purple
when the pH is 9.5 with a maximum wavelength peak at 545 nm; the
solution is stable for more than one week at room temperature. As we
can see in the Figure 1A-B, under the conditions used, only one peak
is highlighted at 545 nm. Further, in order to evaluate the sensitivity
of the modified dye, the MTT-formazan (Sigma) was used as reference
standard and with the formazan enhancer a 1/10 of the initial quantity
was detected with a comparable absorbance value. In the absence
of NaOH 7N no peaks were detected in presence of MTT-formazan
(Figure 1C) 50 µg or (Figure 1D) 500 µg in a range of 400-700 nm
wavelenght. The absorbance of the converted dye was detected with
an Ultraspec 2100 pro spectrophotometer (Amersham Biosciences) in
plastic cuvettes. According to the capability of the formazan to turn to
intense purple after exposition to a strong base milieu, the now abolished
variability among read peaks makes the MTT assay more attractive even if
laboratories cannot use plate reader supply.
Acknowledgments
This work was supported by grants from Ministero dell’Istruzione,
dell’Università e della Ricerca (MIUR, Italia). The authors thanks Dr D.
Sturino for english revision.
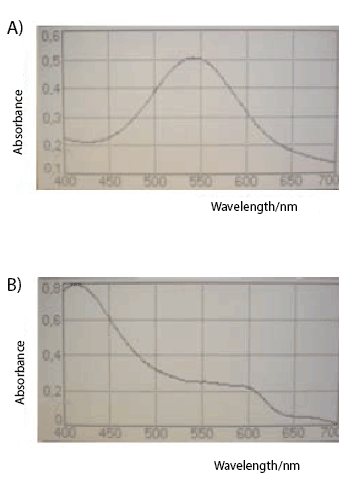
Figure 1: Basic conditions to Formazan improve sensitivity of the MTT assay dye. The enhancer NaOH 7N was added in presence of MTT-formazan (A) 50
µg or (B) 500 µgand a well defined peak was highlighted at 545 nm wavelenght. In the absence of NaOH 7N no peaks were detected in presence of MTTformazan
(C) 50 µg or (D) 500 µg in a range of 400-700 nm wavelength.
Download Provisional PDF Here
Article Information
Article Type: Short Communication
Citation: Perri M, Caroleo MC, Cione E (2016)
Basic Condition to Formazan Improve Sensitivity
of the MTT Colorimetric Assay Dye. J Biochem Analyt Stud 1(1): doi http://dx.doi.org/10.16966/2576-5833.104
Copyright: © 2016 Perri M, et al. This is an
open-access article distributed under the terms
of the Creative Commons Attribution License,
which permits unrestricted use, distribution, and
reproduction in any medium, provided the original
author and source are credited.
Publication history:
Received date: 30 Mar 2016
Accepted date: 10
May 2016
Published date: 16 May 2016


