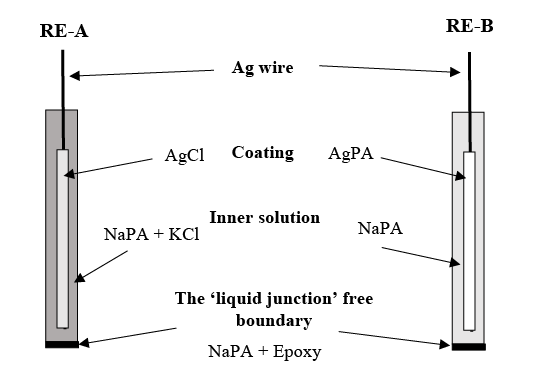
Figure 1: The structures of two reference electrodes.

Liang Wang1,2* Cheng Fang1,2 Ying Cheng1,2 Dane Lamb1,2 Zuliang Chen1,2 Mallavarapu Megharaj1,2 Ravendra Naidu1,2
1Global Centre for Environmental Remediation, Faculty of Science and Information Technology, University of Newcastle, Callaghan, Australia*Corresponding author: Liang Wang, Global Centre for Environmental Remediation, Faculty of Science and Information Technology, University of Newcastle, Callaghan, Australia, E-mail: Liang.Wang@newcastle.edu.au
Sodium polyacrylate (NaPA) is not only well known for the high water absorbability, but also for its electrochemical performance. As knowing NaPA, which is normally used as a polymer binder or cupping reagent, is an anionic polyelectrolyte with negative charge. In this study, NaPA is investigated to develop an Ag/AgPA internal redox system (IRS) for practical reference electrodes (REs). Moreover, the ‘waterlock’ property of NaPA was also applied to lock and gelatinize the inner solution to prevent liquid junction (LJ) between the RE’s inner solution and testing samples. This type of RE has advantages of low cost, easy construction and are suitable for general utilization. The characteristics, including stability, reproducibility and the influence of ionic strength, were studied and validated.
Ag/Ag+ redox system; Reference electrode; Sodium polyacrylate; Liquid junction-free; Solid state electrode
Reference electrodes (REs) are utilized in potentiometry to provide a stable potential as reference in order to measure potential changes of the working electrodes, such as ion-selective electrodes [1-6]. REs which contain Ag/Ag+, i.e. Ag/AgCl, as the IRS, have advantages of good stability and structure simplicity [1,2]. However, the conventional Ag/AgCl REs are normally porous LJ-based and the testing solution will be influenced by Ag+, which will bring in the problems, such as sample contamination, electrolyte loss, and ionic strength variation of the sample [2]. Furthermore, such REs are cumbersome to use and maintain and difficult to miniaturize. Recently, LJ-free Ag/Ag+ REs based on PVC membrane with RTILs (Room Temperature Ionic Liquid) as a sample boundary have been widely studied [7-12]. Huber and Roling [12] briefly summarized some of the studies and applications. These types of membrane-junction REs have the advantage of miniaturisation, however, to synthesize the chemicals for most of RTILs are costly and complicated.
Alternatively LJ problems can be solved simply by employing NaPA, which has lower cost and easier-to-make compared to the REs with PVC membrane with HRTIL. NaPA is an anionic polyelectrolyte with negatively charged carboxylic groups in the main chain. Furthermore, as a superabsorbent hydrogel, NaPA can hold 99.2% deionised water and 91% electrolyte solution [13]. Recently, NaPA has been utilized as a capping reagent [14-18] and polymer binder [19,20] in many applications. For instance, Inaba and co-workers [18] applied NaPA as a capping reagent for preparing cubic Pt nanoparticles. Komaba et al. [20] applied NaPA as a polymer binder of the Si-graphite composite electrode in rechargeable li-batteries. Additionally, the ‘water lock’ property can be applied to hold and gelatinize the inner solution for the REs. For the purpose of miniaturisation and solidification. In this study, NaPA was applied to hold the saturated potassium chloride (KCl) solution to generate a gelatinized Ag/AgCl IRS for RE. Moreover, since NaPA has high electronic conductivity, the mixture with epoxy was applied to create the LJ free boundary between RE’s IRS and testing samples. Alternatively, instead of using saturated KCl solution, deionized water was applied in this study, to create a new RE IRS based on Ag/AgPA using NaPA, and silver wire. The characteristics of these REs were investigated and compared with a commercial Ag/AgCl RE.
The structures of the two Ag/Ag+ REs: 1) RE-A, the IRS was based on Ag/AgCl. In advance, AgCl was electrodeposited onto the silver wire (5 cm length, 0.5 mm, 99.99%, Aldrich) and covered 2 cm length of the Ag wire from one end, using CHI660B electrochemical workstation (CH Instruments Inc., USA) with a conventional three-electrode cell. This three-electrode cell contains a commercial Ag/AgCl (Aldrich) reference electrode, a platinum wire (0.2 mm, Sigma) counter electrode, and the Ag wire was connected as a working electrode. During the decomposition, the Ag wire was treated with a constant potential at 2.0 V for 60 seconds into a 3 mol L-1 potassium chloride (KCl, Fluka) standard solution. For the RE’s inner filling: 3 mol L-1 KCl was locked and gelatinized using sodium polyacrylate salt (Aldrich) at 10:1 wt. ratio, which immersed a 3 cm length of the silver wire from the AgCl coating side, and filled up in a 10-100 µL pipet tip (sigma)’s chamber. 2 mm air dried epoxy (Araldite) was used to seal at the signal transferring side of the chamber with a 2 cm length of Ag wire exposed outside in order to connect to the potential data logging system. The ‘liquid junction’ free boundary between the testing solution and RE’s internal redox system, was made with 2 mm air dried mixture of epoxy and gelatinized NaPA at 1:1 wt. ratio. The NaPA in this mixture was firstly gelatinized with deionized water (Milli Q plus System, with 18.2 MΩ cm-1 resistivity) at a 1:50 wt. ratio. 2) RE-B, instead of using KCl solution to create an Ag/AgCl IRS, deionized water was directly apply to gelatinize the sodium polyacrylate at a 20:1 wt. ratio, to create an Ag/AgPA redox system. AgPA was pre-electrodeposited onto the silver wire with the same procedure as REA, except for treating the Ag wire with a 2.0 V potential constantly for 300 seconds. The RE-B has the same sealing and boundary structure at both sides as RE-A. The structures of these two reference electrodes are shown in Figure 1.

Figure 1: The structures of two reference electrodes.
The potentiometric system consisted of a pH amplifier (AD Instruments Co.) applied as signal amplifiers while the Power Lab (AD Instruments Co.) served to interface the computer with the amplifiers. Chart5 for Windows software (ADInstruments Co.) was used for the data acquisition and filtering phases. Data processing and analysis was undertaken in Matlab R2012b using the statistical analysis tools. Microsoft Excel 2010 was applied for data recording and searching the regression lines. A 10 Hz low-pass digital filter eliminated the noise caused by signal granularity generated by electrodes. One magnetic stirrer (IEC, C876083V) with a spin bar, and several beakers (Schott Duran 250 mL) were used for sample and standard preparation. Solid-state ion selective electrodes (ISEs), includes Na, K, Ca and Mg, were applied in this study. A customized double-junction Ag/AgCl electrode (Model 900200, Orion) was also applied as a standard RE for testing.
The solutions were all made by successive 10-fold dilutions from their 1 M standard solution. The pH values of the testing solutions were in the range of 5.5 to 6.5 (measured by an Orion pH electrode). All reagents employed were of analytical grade (Fluka) and used directly without further purification, unless otherwise stated. Deionized water was utilized in all experiments including the preparation of all aqueous solutions. Standards and samples containing various combinations of each ion were directly measured using the standard addition method. All measurements were carried out at the same temperature (22°C) in triplicate and the average values reported for processing. The final electrode potential was only recorded when stable, i.e. at a constant potential of ± 0.05 mV, over 30 seconds. After each measurement, all containers and electrodes were carefully rinsed with deionized water and blotted dry with tissue paper to prevent electrode cross-contamination.
For testing the temporal reproducibility of the REs (RE-A and REB), both of the REs were continuously immersed in the 3 M KCl and the potential difference against the customized RE for 24 hours (n=3), respectively. The potential drift of both REs are illustrated in Figure 2. It is obviously that the potential values of both REs were keep drifting down over 24 hours Figure 1. However, both of the REs achieved stability in the following time. Comparing with the two REs, the potential values of RE-A were drifiting significantly in the first five hours, from -5 mV to below -10 mV. After that the reading became steady with around a 2 mV difference. While the potential values of RE-B were still drifitng down gradually until the 15th hour, from above -15 mV to near -25 mV. Acknowledging this phenomenon, both the AgCl (RE-A) and AgPA (RE-B) were preelectrodeposited onto the silver wire using a electrochemical workstation. Nevertheless, it took a longer time to electro deposit AgPA (RE-B) onto the Ag wire because the NaPA can be gelatinized and liquefied with larger amount of water. Hence RE-B has less salt in its IRS, and might affect the performance of the RE-B.
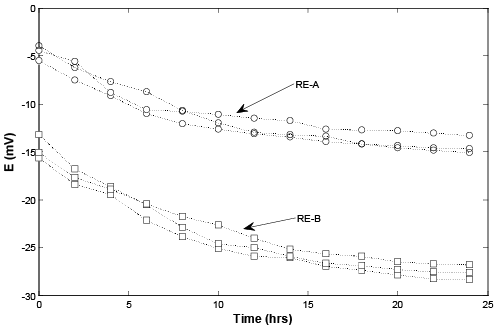
Figure 2: Temporal reproducibility, the first 24 hours by continuously triplicated measuring the solution of 3M KCl (○: RE-A, □: RE-B)
Following the stability testing method, which introduced by Mamińska et al. [2], the stability of the REs were measured triplicate using various concentration levels of KCl, KNO3 , ranging of from 1 µM to 10 mM. A background buffer solution of 0.1 M of Na2 SO4 was utilized to provide strong ionic strength support. The stability of the two REs (RE-A and RE-B) in the solutions with various concentration levels of KCl and KNO3 are presented in Figure 3.
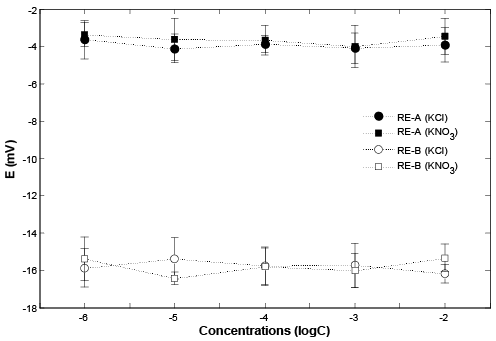
Figure 3: The stability of the two REs: RE-A and RE-B, in the solutions with various concentration levels of KCl and KNO3 (mol/L), background solution: 0.1M Na2SO4.
As mentioned, each sample was measured in triplicate with a 0.1 M of Na2 SO4 background buffer solution. The standard deviations (STD) are also shown in Figure 3. It is obvious that both REs have reasonable stabilities against the customized RE. Both RE-A and RE-B were applied as reference electrodes with different ISEs to measure the various concentration level of their standard solutions. The measurement results were compared using the commercial RE. As shown in (figure 4), expecting the different starting potential values, the detection limits and linear detection ranges for each ISE was maintained, no matter which RE was used. Based on the STDs shown in (figure 4), both RE-A and RE-B had similar performances as the commercial RE.
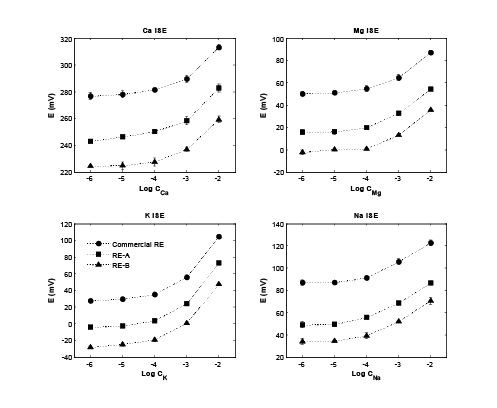
Figure 4: ISEs cooperated with different REs to measure their standard solutions
Both REs (RE-A and RE-B) were developed based on NaPA. RE-A was using NaPA to lock the inner solution KCl and create an Ag/AgCl IRS. RE-B applied NaPA to directly create an Ag/AgPA IRS. Both REs were using NaPA with epoxy to create the boundary between IRS and testing solutions. Furthermore, both AgCl and AgPA were pre-electrodeposited onto the silver wire, using an electrochemical workstation. However, as mentioned, it took longer to electro deposit AgPA onto the Ag wire. This is because the NaPA can be gelatinized and liquefied only with a large amount of water; hence it will dilute the salt and longer electro deposition period, and might also affect the performance of the Ag/ AgPA IRS. Therefore, creating an Ag/AgPA IRS in a more effective way should be considered in the future studies. Scanning Electron Microscope (SEMs) are widely applied to study the surface morphology and structure for electrochemistry, such as electro depositions, where the surface morphology and structure of REs are critical aspects that may influence the electrode properties such as the adhesion, offset potential, and stability. In this manuscript we only focused on the new practical way to make solid-state reference electrodes and the application of new REs. The analysis of RE’s characterizations will be progressed in the future to detail the fabrication processes and reveal the influence to surface characteristics on the stability of different REs.
The authors would like to thank the Cooperative Research Centre for Contamination Assessment and Remediation of the Environment (CERAR) and the University of South Australia (UniSA) for making this research possible. This research was funded by CRC CARE Pty Ltd. The research was conducted in the laboratories of CERAR at UniSA.
Download Provisional PDF Here
Article Type: Research Article
Citation: Wang L, Fang C, Cheng Y, Lamb D, Chen Z, et al. (2016) A Practical Way to Make Solid-State Reference Electrodes. J Biochem Analyt Stud 1(1): doi http://dx.doi.org/10.16966/2576-5833.101
Copyright: © 2016 Wang L, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access