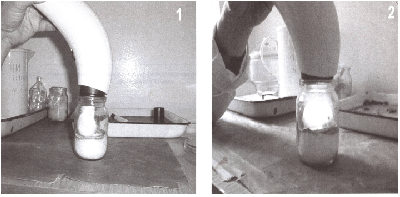
Figure 1: Irradiation with polarized light of laboratory animals (white mice) in the head (1) and in the back (2).
Divocha VA1* Lagoda OV1 Basarab YA2
1SE Ukrainian Research Institute for Medicine of Transport, Odessa, Ukraine*Corresponding author: Divocha VA, Ukrainian scientific research institute of medicine of transport, Odessa, Ukraine, E-mail: divocha09@ukr.net
Studied the effect of polarized light on survival of laboratory animals (mice) infected with a lethal dose of influenza virus A. Irradiation PAYLERlight animals (the entire surface of the back of mice) suspended development of influenza infection and increased defenses of the organism.
Influenza A virus; Polarized light; Proteinase-inhibitors system; White mice
Advantages of influence of solar beams at different diseases of the person are known since ancient times. The modern light theory is not the new theory. In 1903 to the Danish physicist N.R. Finsen was awarded the Nobel Prize in the field of physiology and medicine for his works bound to therapy by light and discovering of a new trend in medicine. By the Russian scientists K.A.Samojlova, K.D.Obolenska, A.V.Volodina, etc. it is shown, that at the expense of penetrating infiltration of polarised light through a skin, descends non-invasive irradiating of formulated elements of a blood. They prove that the direct photo modification which results at an irradiating of 1-3 % of volume in effect generalisation in all volume of a circulating blood. Peroxide oxidation in membranes of erythrocytes drops, and such effect is conserved within 24 hours after unitary influence. In leucocytes (at the expense of described above the mechanism of neogenesis of membranous function and the energy balance) production of antibodies is strengthening, receptor (in relation to foreign agents) and immune - mediator functions are recovering. Production of immunoglobulin, phagocytal activity of cellular elements strengthen, duration of their functioning is prolonged. Linearly polarised light stimulates immunocompetent cells. There is a restoration and stimulation of immune system of an organism and consequently anti-infectious and its anti-virus possibilities raise, the local anti inflammatory effect educes.
It was known that in the cells of animals and humans constantly going various biochemical and physiological processes. Condition of the body depended on various vital sources of energy: light, air, water, food and positive electromagnetic waves (sunlight), coming from the environment. Provided that the polarized light (similar to sunlight) positively affects the body and used in the treatment of various diseases [1-5].
The aim was to study the effect of polarized light on the survival rate of laboratory animals, infected with a lethal dose of influenza A virus.
Influenza virus A/PR/8/34, adapted to lung tissue of white mice and 5 passages had been conducted. All mice were waited only at the start of the experiment. All animals in 1st group died on 5-th day after infection. Homogenized lungs of the dead mice used for determination of proteinase activity and hemagglutination titer. The infection titer of influenza virus A was 7 lg EID50/0.2 ml, titer of hemagglutinin (HA) - 1:64, white mice (line Balbc) weighing 13-14 gr. (40 pcs). Infestation of animal with influenza A virus performed intranasal in volume 0.05 ml (under light ether anesthesia) in dilution of 10-2, in the infectious dose of 20 LD50 virus. This dose provoked 100% mortality of animals on the 6th day after infestation. Animals were divided into 4 groups of 10 mice in the group. 1st group of animals were infected intranasal with a lethal dose of influenza A virus (control of the virus).
2nd group received a lethal dose of influenza virus A in the same parameters as the first group, but is undergoing treatment by Pilar-light. Irradiation was carried out over the entire surface of the animal from the back (Figure 1). Each mouse received 11 irradiation sessions to 6 minutes on the session.

Figure 1: Irradiation with polarized light of laboratory animals (white mice) in the head (1) and in the back (2).
The 3rd group animals received only sessions Pilar-light (11 sessions for each mouse). The 4th group animals received saline solution, which was diluted influenza A virus (animals’ control).
The method is based on qualitative reaction to arginine, formed by the hydrolysis of protamine and histone and non-settleable 20% trichloroacetic acid.
Differences proteinase activity values between control and experimental groups of animals were statistically significant p<0.05. Statistical calculation of results was used in “Microsoft Excell” by general approach.
The activity of trypsin proteinase was determined by method K.I. Veremeyenko [6], the modified S.V. Vovchuk [7]. Protein was determined by O. Lowry [8]. Determination the trypsin of inhibitor-proteinase was performed by A.P. Levitsky [9].
In the 15th day after infection, all animals which were still alive, put to sleep, opened and lungs blood had been taken. Organs were washed 3 times in cold 0.01 M phosphate buffer solution (pH 7.5), crushed with scissors, with a sterile glass triturated in cold sterile mortar, suspended in phosphate buffer (pH 7.5 (1 lung in 1 ml), homogenized by ultrasound in mode #7 using device Hith Infenicity ultrasonic Procession and centrifuged at 104 revol/min. in the centrifuge, Sorval Instruments company, Rotor SS-34 for 1 hour (t+4° C).
The supernatant and the blood serum were used to determine proteinase and hemagglutinating activity and total protein. Infectious virus titers in lungs of infected animals was determined by infecting 9-10- day chick embryos and expressed in lg EID50/0,2 ml. Hemagglutination reaction was carried out by the usual method.
The supernatant and the blood serum were used to determine proteinase and hemagglutinating activity and total protein. Infectious virus titers in lungs of infected animals was determined by infecting 9-10- day chick embryos and expressed in lg EID50/0,2 ml. Hemagglutination reaction was carried out by the usual method.
For the treatment of animals infected with a lethal dose of influenza A virus have used polarized, non-coherent, polychromatic (Pilar) light with a wavelength of 400-2000 nm with light energy of 2.4 J/cm2 during 6 min. one time in the day, the general course of 11 sessions. In the 1st day after infection mice were irradiated at 1 and 6 hours.
Research results were presented in the table 1. 100% mortality of animals of the 1st group (control of influenza A virus) was observed in the 5th day after infection. In the 2nd group of animals (influenza virus A + treatment with polarized light) in the 14th day after infection, 50% mice remained alive. Observations showed that after 2 days after infection, animals were lethargic, bad eating. Starting with the 4th day, these symptoms disappeared. The 3rd group of animals received only irradiation of polarized light was active, and all animals survived. All animals 4th group received saline and served as controls for animals themselves, also survived.
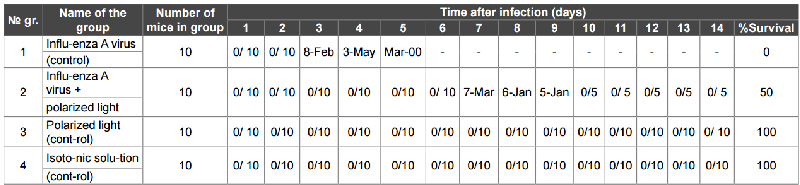
Table 1: Survival of mice infected with a lethal dose of influenza virus A/PR/8/34 with polarized light
Note: the numerator - the dead mouse; the denominator - the surviving mouse.
During the determination (in the blood serum of mice) of the activity of trypsin-like proteases, the protein content and hemagglutinating activity after 14 days after infection (Table 2) we found out:
№ gr. |
Name of the group |
Proteinase activity in lungs of mice, units/ml* |
Proteinase activity in serum blood in mice, units/ml* (mixed sample) |
1. |
Influenza A virus |
125.5 ± 49.5* |
387 |
2. |
Influenza A virus + polarized light |
182.0 ± 49.25* |
80 |
3. |
Polarized light |
192.8 ± 50.93* |
267 |
4. |
Isotonic solution (control) |
229 ± 53.71* |
74 |
Table 2: Changing proteinase activity in mice, infected with influenza virus A/PR/8/34, after exposure to polarized light (n = 24, M ± m)
Note: PA - Proteinase activity in lungs of mice, enzyme activity - trypsin-like proteinases in units/ml. For 1 unit of activity defined as the amount of enzyme causing formation of 1 micromole of arginine in 1 min. incubation (p ≤ 0.05).
In the 1st group of animals’ proteinase activity in lungs is significantly suppressed and decreased by 50% compare to the 4th group (control) – 125.5 ± 49.5 and 229 ± 53.17 un/ml, respectively.
In the blood serum of mice at the same time the high content of proteinase - Note: PA - Proteinase activity in lungs of mice, enzyme activity - trypsin-like proteinases in units/ml. For 1 unit of activity defined as the amount of enzyme causing formation of 1 micromole of arginine in 1 min. incubation (p ≤ 0.05). Compared with the 4th group (control) - 74 ± 6.4 un/ml. Under the influence of polarized light in the 3rd group of healthy animals showed a slight reduction of proteinase activity in lungs – 192.8 ± 50.93 un/ml compared with the 4th group - 229 ± 53.71 un/ml and a high content of proteinase in blood serum of mice - 267 and 74 un/ml (group 4). In the treatment with polarized light of mice infected with a lethal dose of influenza A virus (group 2), found out that the proteinase activity in lungs of mice was at the same level (182.0 ± 49.25 un/ml), and that animals 3rd group (192.8 ± 50.93 un/ml), which received irradiation with polarized light, but substantially higher than the 1st group, who were not treatment (125.5 ± 49.5 un/ml). In blood serum of the mice proteinase activity was lower in the 2nd and 4th groups of animals.
Hemagglutinating activity in lungs and blood serum in the 3rd and 4th groups of animals has not been determined. It is important that in the 2nd group of animals received a lethal dose of influenza A virus with polarized light therapy titer influenza A virus after 14 days infection (the entire observation period) was 1:2.
Due to the fact that the polarized light irradiation, we performed when influenza A virus penetrated into the cell, and after 6 hours of exposure, when the first multiplication cycle of the virus and output in the extracellular space, we hypothesized that polarized light destroyed enzymes of cells which are responsible for the cleavage of hemagglutinin (HA) of influenza A virus into 2 subunits HA1 and HA2, which are responsible for the penetration and proliferation of influenza A virus in the cell [10,11].
Thus, irradiation of animals by polarized light (the entire surface of the back of mice) infected with a lethal dose of influenza virus A/PR/8/34, suspended the development of viral influenza infection, and white mice recover.
The treatment with polarized light of white mice, infected with a lethal dose of influenza A virus, suspended the rate of reproduction of the influenza A virus and its activity in the body. Infectious and hemagglutinating activity was determined in small titers. Polarized light did not destroy the influenza A virus in the host organism, but only hindered its reproduction in animals. During this period, recovery was protective inhibitory activity in mice and they survived.
Download Provisional PDF Here
Article Type: Research Article
Citation: Divocha VA (2016) The Influence of Polarized Light in Defenses of Mice Infected with a Lethal Dose of Influenza A Virus. Autoimmun Infec Dis 3(2): doi http://dx.doi.org/10.16966/2470-1025.121
Copyright: © 2016 Divocha VA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access