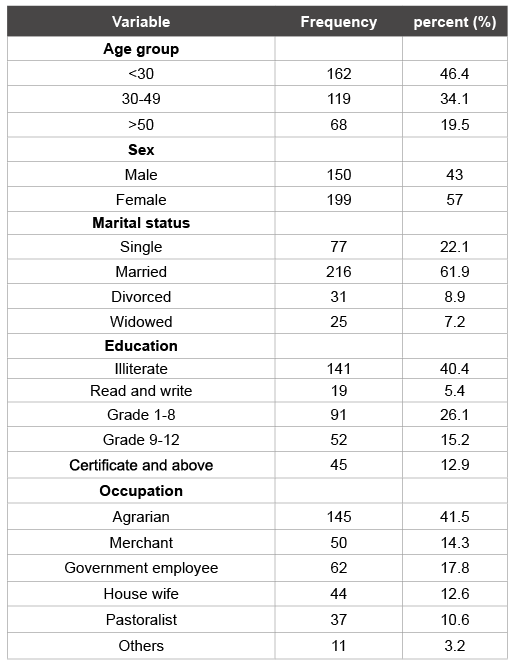
Table 1: Frequency of socio-demographic characteristics of participants, Jinka Zonal Hospital, Debub Omo Zone, SNNPR, 2013 (n=349)
Getnet Hailu* Kassu Desta Fisihatsion Tadesse
Ethiopian Public Health Institute, Gulele Sub city, Addis Ababa, Ethiopia*Corresponding author: Getnet Hailu, Ethiopian Public Health Institute, Gulele Sub city, Arbegnoch street P.O.Box 1242 Addis Ababa, Ethiopia, Tel: +251112788648; E-mail: getnethailu21@gmail.com
Background: Nearly 50% of the world’s population is estimated to be infected with Helicobacter pylori, but the prevalence varies greatly among countries and population groups within the same country. The overall prevalence of Helicobacter pylori infection is strongly correlated with socioeconomic conditions.
Objective: To determine the prevalence and the possible risk factors for Helicobacter pylori infection among adults.
Methods: A hospital based cross-sectional study was conducted from December 2012 to February 2013among 349 adults. All Stool specimens were screened for fecal Helicobacter pylori antigen. Besides, all study units were interviewed using a structured questionnaire.
Results: A total of 150(43%) males and 199(57%) females were involved in the study. The age of participants were ranged between 20-89 years with a mean age of 36.7 ± 14.7 and median 32 years. The overall prevalence of Helicobacter pylori infection was 50.7% (177/349). Helicobacter pylori infection was positively associated with those whose occupation were agrarians (OR=1.85(95% CI 1.02-3.39, p=0.045)); being male (OR=1.98(95% CI 1.42-3.29, p=0.011)), more than 5 persons living in the same house, [(OR=1.53(95%CI 1.00-2.34, p=0.048)]; those practiced open field defecation/no toilet use, (OR=6.75(95% CI 2.11-21.61), p=0.001); those who never wash their hands after toilet,(OR=2.86(95%CI 1.30- 3.27, p=0.009). But a minimum alcohol consumption was negatively associated with Helicobacter pylori bacteria, (OR=0.39(0.23-0.67, p=0.001).
Conclusion and recommendation: The overall prevalence of Helicobacter pylori infection in Debub Omo Zone was 50.7%. Poor hygienic practices and crowding were positively associated with Helicobacter pylori infection. On the other hand, consumption of little alcohol might protect infected against Helicobacter pylori bacteria. Increasing the awareness of the communities toward good hygienic practices might reduce the transmission of Helicobacter pylori infection.
Helicobacter pylori; Alcohol; Infection; Transmission
Nearly 50% of the world’s population is estimated to be infected with H. pylori, but the prevalence varies greatly among countries and among population groups within the same country. H. pylori is one of the most common infections in humans affecting 30-40% of persons living in the developed, and 80-90% of persons living in the developing world [1,2].
The prevalence among middle-aged adults is over 80 percent in many developing countries, as compared with 20 to 50 percent in industrialized countries. Therefore, overall prevalence is high in developing countries and lower in developed countries and within areas of different countries. There may be similarly wide variations in the prevalence between more affluent urban populations and rural populations. The principal reasons for these variations involve socioeconomic differences between populations [1,2].
H. pylori, the principal species of the genus Helicobacter, was discovered by Warren and Marshall in 1983. This is a small, curved, highly motile, gram-negative bacillus recognized as a chronic colonizer of the human stomach; and known to be one of the most genetically diverse of bacterial species. Biochemically it is urease, catalase and oxidase positive [2-4].
Globally, different strains of H. pylori appear to be associated with differences in virulence, and the resulting interplay with host factors and environmental factors leads to subsequent differences in the expression of disease. Age, ethnicity, gender, geography and socioeconomic status are all factors that influence the incidence and prevalence of H. pylori infection [4].
A lack of proper sanitation, of safe drinking water, and of basic hygiene, as well as poor diets and overcrowding, all play a role in determining the overall prevalence of infection. In general, H. pylori sero-positivity rates increase progressively with age, reflecting a cohort phenomenon. In developing countries, H. pylori infection is markedly more prevalent at younger ages than in developed countries [3,5].
Despite many attempts at discovery, the exact source of infection has yet to be determined. Contaminated food or water sources have been cited as important risks for becoming infected with H. pylori. Laboratory experiments have found that H. pylori may survive up to a week in water [6].
Even though H. pylori infection in humans has been convincingly linked to the development of gastrointestinal (GI) diseases, only a small percentage of colonized individuals will express clinical manifestations. The virulence of the infecting strain is likely to be a major determinant for developing the diseases, although the process is a complex interaction between host and bacteria. A proposed determinant for the outcome of colonization is the ability of the bacterium to attach to the gastric epithelium. In vitro studies have indicated that H. pylori strains express a variety of adhesins that recognize a number of epithelial receptors [7].
One of these receptors is the antigen of the Lewis blood group system that is not only synthesized by erythrocytes and blood vessel endothelium but by secretorial cells of the stomach. Demonstration showed that the blood group antigen Lewis b on the gastric epithelial cells acted as an H. pylori receptors in vitro, and it has further been shown that the Lewis b receptor recognizes the product of the H. pylori babA gene [8].
The principal reservoir of H. pylori is man, but there have been descriptions of infection spread by means of water or uncooked vegetables contaminated with sewage and a host of other factors [2]. The role of domestic animals in the spread of infection still remains unclear. The putative routes of transmission of the organism have been reported to be fecal-oral, oral-oral, and gastric-oral [9].
Once a person is infected, it can persist in the stomach for decades despite a systemic immune response. The reasons for the failure of the immune system to control infection may be attributed to the fact that H. pylori produces chemical components in their cell walls that are very much similar molecules made by the stomach cells of the host. This creates a problem for the immune system, because it is designed to ignore molecules made by the host (self) and to recognize molecules produced by infectious agents (non-self) [10,11].
There are various techniques of detecting H. pylori from specimens. These tests may be invasive or non-invasive. Endoscopy and gastric mucosal biopsy, microscopic examination of histological sections and rapid urease test are forms of invasive test that could be used. Non-invasive tests such as Urea Breath Test (UBT), Enzyme Linked Immuno Sorbent Assay (ELISA), H. pylori stool antigen test (HpSTAR and HpSA), and latex agglutination tests are important tests. Detection of the antigen however gives a more precise result considering the waning nature of antibodies especially after an infection [10,12].
No single agent has been shown to be effective for curing infection in the majority of patients. Because of the increasing problem of antimicrobial resistance, extended (10-14 day) proton pump inhibitor–based regimens with at least 2 drugs (preferably 3 or 4) should be used for treatment [13,14].
The study was conducted at Jinka Zonal Hospital, Debub Omo Zone, Southern Nations, Nationalities and Peoples Region (SNNPR). Jinka Zonal Hospital is the only Zonal Hospital found within the South Omo zone. Patients were come to this Hospital from different health facilities and wored as of the zone.
Hospital based cross-sectional study was conducted from December 2012 to February 2013.
All adults with Upper Gastro-Intestinal (UGI) symptoms who visited the hospital.
All adults with Upper Gastro-Intestinal (UGI) symptoms who visited the Hospital during the study period.
Stool specimen was collected in clean, wide mouth and screw caped containers. Stool antigen test, (Croma test, linear chemicals S.L), is a qualitative immune-chromatography assay for the determination of Helicobacter pylori antigen in fecal samples. The membrane is pre coated with monoclonal antibodies, on the test band region, against H. pylori antigens.
The blood specimen was collected by using capillary tube from figure prick to determine ABO blood group using slide method. ABO blood grouping was determined by testing unknown red cells against known anti A, and anti B antibodies (Croma test, linear chemicals S.L).
Statistical analyses were done using chi-square to evaluate any association between H. pylori infections with different risk factors. Observed differences in data were considered significant and noted in the text if p<0.005 was obtained.
A total of 349 individuals who had upper gastrointestinal symptom were enrolled in this study. The age of participants were ranged between 20 and 89 years with a mean age of 36.7 ± 14.7 and median of 32 years. Of the total study participants, majority, 199(57%) and 216(61.9%) were female and married respectively. Farming 156(44.7%) was the means of livelihood for most study participants. Among the total study participant, 188(53.9%) were residing from rural, 161(46.1%) were protestant and 144(40.4%) were illiterate.
Among 349 study participants, fecal H. pylori antigen was detected in 177(50.7%). The prevalence of H. pylori infection among age group less than 30 years (48.1%) was almost similar to age group 30-49 years old (47.9%). While there was an increase prevalence of H. pylori infection was recorded among participants aged ≥ 50years (61.8%). High prevalence of H. pylori infection were noted among pastoralists, 25/37(67.6%) and Merchants, 30/50(60%) (Table 1).

Table 1: Frequency of socio-demographic characteristics of participants, Jinka Zonal Hospital, Debub Omo Zone, SNNPR, 2013 (n=349)
The prevalence of H. pylori was assessed for any association with the socio-demographic data, health character, living style and hygienic practices of the respondents. Of the total males participants, 91/149 (61%) were positive for H. pylori stool antigen compared with females 86/200 (43%). The overall OR of H. pylori infection for males compared with females was OR=1.98 (95%CI 1.42-3.29, p=0.011). Hence, males are more likely to be infected with H. pylori bacteria than females. Statistical significant association was noted between H. pylori infection and occupation (p<0.05) of the study participants. Even though the prevalence of H. pylori infection were high among pastoralists, 25/37(67.6%) and Merchants, 30/50(60%), there was no statistical significance association were observed. Clearing the possible confounding factors, a multiple logistic regression model showed that statistical significant difference was recorded between agrarian and fecal H. pylori Antigen positivity. I.e. OR=1.85(95%CI (1.02-3.39, p=0.045). Therefore, the odds of having H. pylori infection among agrarians were high (Table 2).
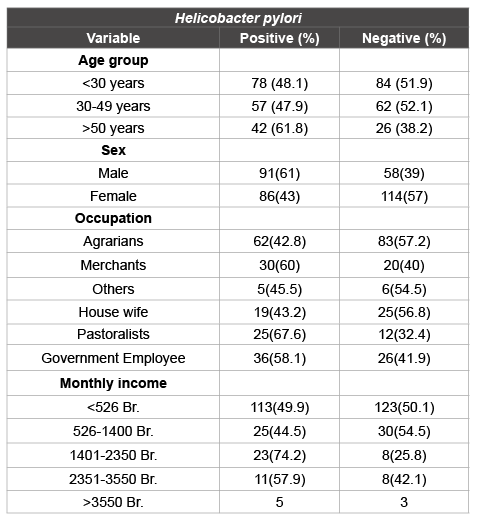
Table 2: Distribution of Helicobacter pylori infection with socio-demographic characters among participants at Jinka Zonal Hospital, Debub Omo Zone, SNNPR, 2013 (n=349)
From the total study participants, 20 out of 31 (64.5%) AB blood group, 43 out of 85 (50.6%) A blood group, 52 out of 104 (50%) B blood groups were positive for H. pylori stool antigen respectively (Table 3). However, no statistical significant associations were observed between fecal H. pylori infection and blood group (p>0.05).
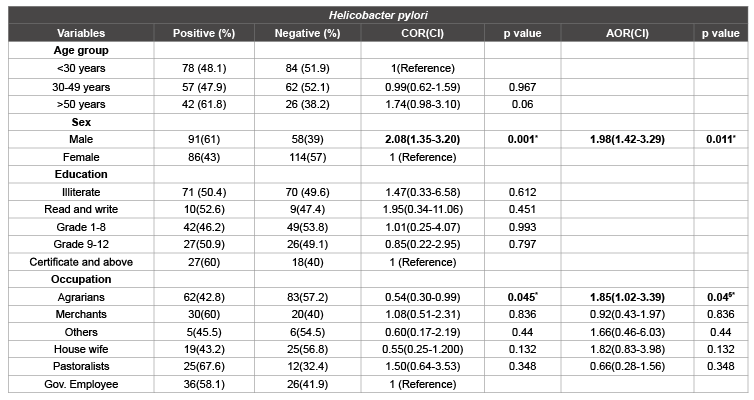
Table 3: The prevalence of H. pylori was assessed for any association with the socio-demographic data, health character, living style and hygienic
practices of the respondents
COR: Crude Odds Ratio; AOR: Adjusted odds ratio; 95% CI: 95% confidence interval; OFD: Open Field Defecation; VIP: Ventilated Improve Pit latrine; *
significance difference
Statistical significant association was detected between H. pylori infection and alcohol consumption (p<0.05). In addition, a multiple logistic regression clearly noted that alcohol consumption lee than once a weak can reduce H. pylori infection by 61% as compared to those who never drank alcohol [OR=0.39(95% CI 0.23-0.67, p=0.001)]. But no association was distinguished between alcohol consumption more than once a week and H. pylori infection. In conclusion, alcohol consumption is an independent predictor for H. pylori infection.
The prevalence of H. pylori infection was 56.1% and 43.9% among the number of persons living in the same house containing <5 persons and ≥ 5 persons respectively. Statistical significant association was detected with H. pylori infection (p<0.05). A multiple logistic regression showed that association was detected between H. pylori infection and number of persons living in the same house containing ≥ 5 persons [OR=1.53(95%CI 1.10-2.34, p=0.048)]. Hence, crowded families are more likely to be infected with H. pylori bacteria than families contain less than 5 persons in the house. Therefore, crowding is an independent predictor of H. pylori infection.
Of the total study participants, 315 used either private or public latrine. But the rest practiced open field defecation/no toilet/. Out of 24 individuals who used public latrine, seven (29.8%) were positive for H. pylori fecal antigen. While 145(49.8%) individuals who used private latrine were also infected. In addition, among the participants who practiced open field defecation/no toilet/, 25(73.5%) were positive for H. pylori stool antigen tests. Statistically significant association was detected between latrine usage and H. pylori infection (p<0.05) (Table 4).
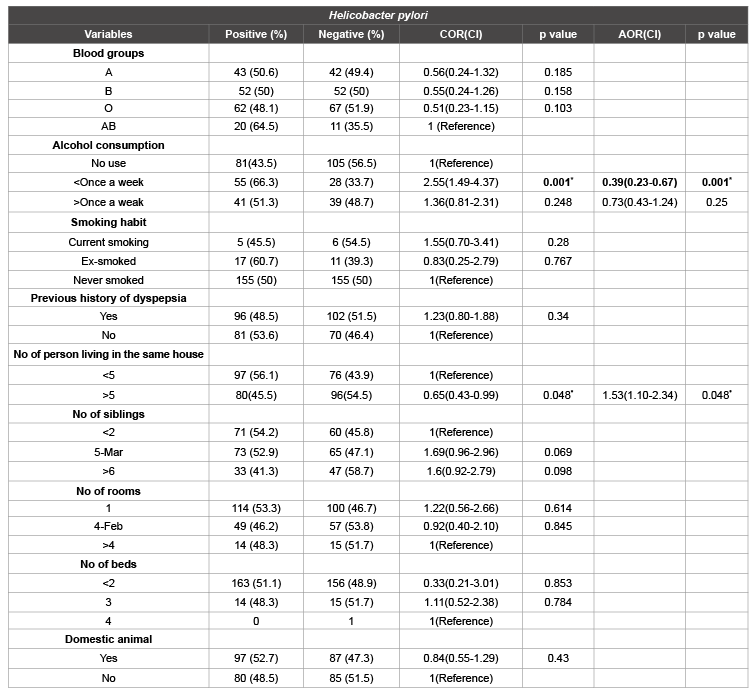
Table 4: Distribution of H. pylori infection and association with different medical character and life style of the respondents at Jinka Zonal Hospital, Debub
Omo Zone, SNNPR, 2013 n=349
COR: crude odds ratio; AOR: Adjusted odds ratio; 95% CI: 95% confidence interval; OFD: Open Field Defecation; VIP; Ventilated Improve Pit latrine; *
significance difference
Generally, multiple logistic regression model noted that, the odds of H. pylori infection among individuals who practiced open field defecation /no toilet/ and used public latrine were 6.8 and 2.8 times higher than those used private latrine, [OR=6.75(95%CI 2.11-21.6, p=0.001) and 2.80(95%CI 1.26-6.20, p=0.011], respectively. Therefore, toilet use is another independent predictor of H. pylori infection.
Among participants who had never washed their hands after toilet, 32 (72.7%) were positive for H. pylori infection and it has statistically significant association with fecal H. pylori antigen positivity (p<0.05). Multiple logistic regression model showed, [OR=2.86(95%CI 1.30-3.27, p=0.009]. Hence, the odds of being infected by H. pylori bacteria among individuals who never wash their hands after toilet is 2.86 times higher than those wash their hands always after toilet use. Therefore, this hygienic practice is an independent predictor of H. pylori infection.
Among the different variables, no statistical significant difference were observed in education level, monthly income, religion, smoking habit, residence, previous history of dyspepsia, number of siblings, number of beds, domestic animals, toilet type, water source, hand wash before meal, habit of raw vegetable, fruit and raw milk consumption with H. pylori infection.
A total of 150(43%) males and 199(57%) females were involved in the study. The age of participants were ranged between 20-89 years with a mean age of 36.7 ± 14.7 and median 32 years. Among 349 study participants, 177(50.7%) were positive for fecal H. pylori antigen. H. pylori infection were positively associated with males (AOR=1.98(95%CI 1.42- 3.29, p=0.011), whose occupation were agrarians (AOR=1.85(95%CI 1.02-3.39, p=0.045); number of persons living in the same house ( ≥ 5 persons)[(AOR=1.53(95%CI 1.10-2.34, p=0.048)]; those practiced open field defecation/no toilet use/ (AOR=6.75(95%CI 2.11-21.61), p=0.001); those who never wash their hands after toilet use(AOR=2.86(95%CI 1.30- 3.27, p=0.009). But negatively associated with individuals who drank alcohol less than once a week (AOR=0.39(0.23-0.67, p=0.001) (Table 5).
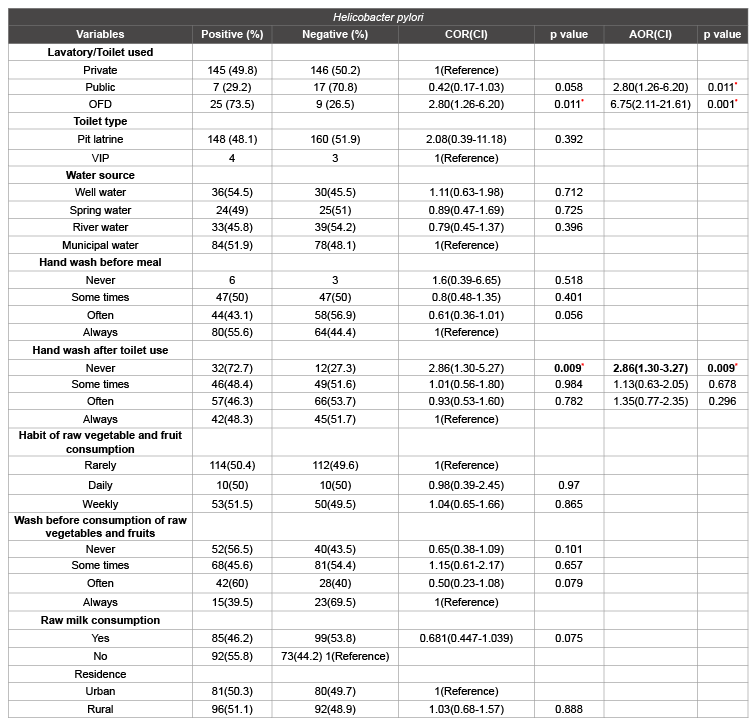
Table 5: Distribution of H. pylori infection and association with different hygienic practices of the respondents at Jinka Zonal Hospital, Debub Omo Zone,
SNNPR, 2013 n=349
COR: crude odds ratio; AOR: Adjusted odds ratio; 95% CI: 95% Confidence interval; OFD: Open Field Defecation; VIP: Ventilated Improve Pit latrine; *
significance difference
Infection with H. pylori occurs worldwide, but the prevalence varies greatly among countries and among population groups within the same country. The mode of H. pylori transmission however remains controversial [15].
Compared to similar studies, this work is, to our knowledge, the first and the largest on H. pylori prevalence and its risk factors in a selected group of population at Debub Omo Zone. In this study, the HpSA test was used to detect H. pylori antigen. The test is a reliable non-invasive method for detecting H. pylori antigens in stool specimens because active infection can be delineated. This method has also been shown to have high sensitivity and a positive predictive value for the detection of H. pylori infection [2,12].
In this study the overall prevalence of H. pylori infection was 50.7% (177 of 349), which was less than studies done in Addis Ababa (89%), Gondor (85.6%), Arbaminch (73%), and Hawassa (62.3%). The possible explanation is that the prevalence of H. pylori infection varies among countries and among population groups within the same country. This is may be due to the above studies was done in different age groups and used different study design and sample size [12,15-17].
Another explanation may be the different sensitivity of the method of laboratory diagnosis. Serological testing was used in the above studies whereas H. pylori stool antigen was used in this study. This test detects active infection, while serology does not differentiate between current and past infection. Therefore, the use of serology may lead to an overestimation of prevalence by including subjects who had been infected but were cured prior to testing [15].
This study showed that statistical significant difference was obtained between prevalence of H. pylori infection and gender. i.e. H. pylori colonization was higher in males OR=1.98(95%CI 1.42-3.29, p=0.011) than in females. This finding is in accordance with many reports from the literature where males were found to have significantly higher infection rates than females [5,18,19].
Several studies illustrate that immunological differences exist between the sexes that may underlie increased infection in males. Females typically have higher immune responses than males.
It was not found statistically significant association with age group, ethnicity, and marital status, place of residence, monthly income, and education (p>0.05). However, many studies found a strong association between these factors and H. pylori infection, there are also some that did not associate. Increased age was associated with H. pylori infection in Addis Ababa, Gondor, Hawassa, Tanzania and other developing countries. Rural residence was related in Taiwan and Kazakhstan. Studies did not found a significant association between H. pylori infection with ethnicity, low educational level and monthly income in Hawassa, Turky, Mexico and Mato Grosso. All these reports are consistent with the concept that the most important factors influencing the transmission of an infection may differ with geographical location and study population. Therefore, the absence of statistically significant association with these demographic factors in this study (p>0.05) might be due to similar grounds with the above concept including difference in sample size [12,20-26].
In our study, we found a higher prevalence of H. pylori infection in those having small number rooms (53.3%) and an increase of people sharing the same bed (51.1%).But no significant association was noted with the infection. This difference may be due to the small sample size used in our study.
In this study, the prevalence of H. pylori infection was high in those who practiced open field defecation/no toilet/ i.e. 73% and public latrine users 29.2% respectively. Statistical significance associations were obtained between H. pylori infection and latrine usage. Therefore, individuals who use private latrine was less likely infected with H. pylori than the others. No identical data were, however, available on that aspect for comparison.
The assumption is that, poor personal and environmental hygiene may play a great roll in the transmission of H. pylori bacteria. These findings supports the notion of oral-oral or fecal oral route with or without intermediate vectors of transmission which is thought to be the primary route of transmission [27].
These findings are in accordance with several reports in the literature that assessed the relationship between alcohol consumption and H. pylori infection. A pooled analysis of three studies from Southern Germany, comprising 1410 adults aged 15 to 69, showed that prevalence of current H. pylori infection was lower among subjects who consumed alcohol (34.9%) than among non-drinkers (38.0%), regardless of the type of alcoholic beverages consumed. In 2002, the report of the Bristol Helicobacter Project found a negative association between consumption of wine and beer and H. pylori infection prevalence in 10,537 participants [28,29]. Given the fact that acquisition of H. pylori infection occurs in childhood, the inverse association between alcohol consumption and H. pylori infection would more likely reflect suppression or elimination of the infection by alcohol consumption rather than reduced rates of acquisition of the infection. [28,29].
In this study, high prevalence of H.pylori infection was detected in those who never washed their hands after toilet use (72.7%), this poor hygienic practice increase the odds of infection with H.pylori bacteria by 2.86 times than those who washed always their hand after toilet used. The possible explanation is that poor personal and environmental hygiene contribute to the transmission of the bacterium via feco-oral rout.
Individuals with blood group O were found to be more susceptible to peptic ulcer disease for decades without known cause until the relationship between Lewis antigens and the attachment of H. pylori to gastric mucosa was observed. However, the correlation between H. pylori infection and ABO blood types was not supported in some reports, Addis Ababa, Bahir Dar and Gondor. In this study, although the most prevalent blood group was blood type O (43%), subjects with blood group O didn’t show an increased susceptibility to H. pylori infection than those with other blood groups (p>0.05) [12,16,30-34].
Of the total study participants, 50.7% were positive for fecal Helicobacter pylori antigen. High prevalence was noted among age group ≥ 50 year but no statistically significant association was detected (p>0.05). There was a considerable increase of H. pylori prevalence in males than females and being male had statistical significant association with H. pylori infection. Even though high prevalence were obtained among pastoralists (67.6%) and merchants (60%); statistical significant association were noted between agrarians and H. pylori (OR=1.85(95%CI 1.02-3.39), p=0.045).
Statistically significant positive associations were obtained between H. pylori infections and more than or equals to five persons living in the same house (crowding) and poor hygienic practices. But consumption of little alcohol might protect from being infected by H. pylori bacteria [OR= 0.39(95% CI 0.23-0.67, p=0.001)].
No statistical significant difference were observed in education level, monthly income, religion, smoking habit, previous history of dyspepsia, number of siblings, number of beds, domestic animals, toilet type, water source, hand wash before meal, habit of raw vegetable and fruit consumption, wash before consumption of raw vegetable and fruits and raw milk consumption with H. pylori infection. 57.9 %(202) were positive for at least one of the different intestinal parasites.
Therefore, good hygienic practices may reduce infection with H. pylori bacteria.
Download Provisional PDF Here
Article Type: Research Article
Citation: Hailu G, Desta K, Tadesse F (2016) Prevalence and Risk Factors of Helicobacter pylori among Adults at Jinka Zonal Hospital, Debub Omo Zone, Southwest Ethiopia. Autoimmun Infec Dis 2(2): doi http://dx.doi.org/10.16966/2470-1025.113
Copyright: © 2016 Hailu G et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access