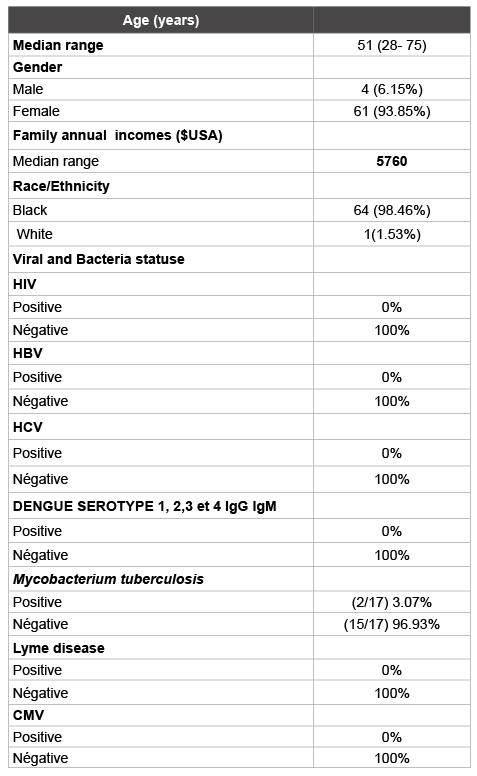
Table 1: Demographic and clinical characteristics of patients with in the study cohort (n = 65)
Sourabié Y1,2* Bazié WW1 Sangaré I1,2 Sirima C1 Ouédraogo MS1,2 Fumoux F4 Traoré Y3
1University Hospital Souro Sanou, 01 BP 676, Bobo, Burkina Faso*Corresponding author: Yacouba Sourabié, Teacher-Researcher in immunology at Higher Institute of Health Sciences, Polytechnic University of Bobo Dioulasso, Hospitalo - University Centre Souro Sanou, Department of Immunology and Hematology, BP 676, Tel: 0022670710325; E-mail: yacourabie@yahoo.fr
Background: Autoimmune diseases have been rarely reported in limited setting country. We aimed to investigate the burden, immunological and clinical diagnosis of autoimmune diseases in Burkina Faso.
Methods: We prospectively enrolled patients with a suspected autoimmune disease at University Hospital SOURO SANOU, in Bobo Dioulasso, BURKINA FASO, between 2012 and 2014 for this cohort study. Demographic characteristics, clinical manifestations, sera autoantibodies determination and plasma viral and bacteria statuse (HBV, HCV, HIV, and Dengue serotype 1, 2, 3 and 4, Mycobacterium tuberculosis and Lyme disease) were determined. All plasma samples were stored at – 80°C when analyzed was not making immediately.
Results: During the three year study period, totally 65 patients with suspicion of autoimmune diseases were diagnosed. The referral diagnosis was right in 17/65 patients (26.2%) among patients in the limited setting country (Burkina Faso). The incidence of autoimmune diseases was 17/73950 (0.023%). There were 4/73950 (0.005%) patients with systemic lupus erythematosus (SLE), 5/73950(0.007%) with rheumatoid arthritis (RA), 2/73950 (0.003 %%) with CREST and Polymyositis, 2/73950 (0.003%) with CREST, 1/73950 (0.001%) with Gougerot Sjögren’s syndrome (SSG), 2/73950 (0.003%) with Thyroiditis of Hashimoto and, 1/73950 (0.001%) with sarcoidosis. The median age at the diagnosis of autoimmune diseases were respectively 51 years (28 -75 years) and male to female ratio of 0.06 (1/16). The plasma viral status (HBV, HCV, CMV and HIV, Lyme disease, Dengue serotype 1, 2, 3 and 4) was negative. Mycobacterium tuberculosis’s cases were 3.07%). The immune suppressor’s treatment again autoimmune diseases were not efficient among 2/17 of patients.
Conclusion: The referral diagnosis was right in 17/65 (26.2%). The incidence was 17/73950 (0.023%) in the limited setting country (Burkina Faso). There was not a significant associated relation between autoimmune diseases and infection; there was not also significant association between autoimmune diseases and hygienic hypothesis.
Autoimmune; Disease; Autoantibody
ANA: Anti-nuclear Antibodies; CENP-B: Centromere protein B; dsDNA: Double-stranded DNA; ELISA: Enzyme-linked Immunosorbent Assay; IFI: Indirect Immunofluorescence Assay; Jo-1: Histidyl-t-RNA synthetase; AID: Autoimmune Disease; RNP70 A,C: Small nuclear ribo nucleoprotein complexes; 70 kDa, A, C polypeptides Scl-70: DNA topoisomerase I; SLE: Systemic lupus erythematosus; SmD: Smith’s antigen (a family of RNA-binding proteins); SSA/Ro: Sjögren’s Syndrome A antigen/small ribonucleoprotein particle (Ro 52 and 60 kDa); SSB/La: Sjögren’s Syndrome B antigen/Lupus antigen, La ribonucleoprotein domain family, member 3 ; U1RNP: U1 nuclear ribonucleoprotein (mixture of recombinant RNP70, A, C); RA: Rheumatoid Arthritis; HT: Hashimoto Thyroiditis
Autoimmune diseases represent a heterogeneous family of chronic, disabling diseases with different natural histories and a wide spectrum of clinical symptoms. These disorders share underlying defects in the immune response leading the body to attack its own organs and tissues. Most of these diseases disproportionately affect women; however, persons of all racial, ethnic, and socioeconomic groups are affected [1,2]. Certain diseases, including systemic lupus erythematosus and scleroderma, are more common in African Americans, whereas others, such as type 1 diabetes and multiple sclerosis, are more common in Caucasians. All ages are affected, with onset from childhood to late adulthood. While many individual autoimmune diseases are rare, collectively they are thought to affect approximately 5 to 8 percent of the United States population – 14 to 22 million persons [3-8]. Because of their chronicity, measured in decades, and their debilitating complications, autoimmune diseases exact high medical and socioeconomic costs. In addition, since autoimmune diseases affect women in their most productive years, their impact on families and society can be substantial. Although comprehensive national data on the incidence, prevalence, and medical and economic impact of autoimmune diseases do not exist in the aggregate, or for the majority of individual autoimmune diseases, the statistics that are available make clear that the impact of these diseases is significant. Some of the available data for specific diseases are highlighted below. The purpose of our study is to establish immunology and clinical strategies for enhancing autoimmune diseases diagnosis in the limited setting country (BURKINA FASO) were incidence, prevalence, morbidity, and mortality of autoimmune diseases remains undiscovered.
Samples analyzed in our study derived from patients who went to hospital for consultation. Patients with autoimmune diseases enrolled in our study received effective immunosuppressors treatment alone or in association with another drug. The study was approved by our National Ethic Committee for health research in Burkina Faso Ouagadougou. Patients participating in this study gave written informed consent.
We prospectively enrolled patients who had a medical and immunology diagnosis of autoimmune diseases at University Hospital SOURO SANOU and another private clinical, in Bobo Dioulasso, BURKINA FASO, between January 2012 and November 2014 for this cohort study. Demographic characteristics, clinical manifestations, sera auto antibodies profile and plasma viral and bacterial statuses (HIV/HBV/HBC/CMV/ Dengue serotype 1, 2, 3 and 4 /Mycobacterium tuberculosis/Lyme disease) were determined.
In our study, autoimmune diseases diagnosis was a combination of autoantibody blood tests-rays, clinical presentation and blood tests that measure nutritional function and inflammation. In addition, a criterion of diagnosis was used to confirm each case of autoimmune disease without IFI method; the sera autoantibody profile of the patient represents an important prerequisite for the clinical diagnosis. Indeed, positive results for ANA and the presence of anti–double-stranded native DNA (antidsDNA) or anti-Sm antibodies constitute 2 of the 11 criteria for the diagnosis of systemic lupus erythematosus (SLE) [2,9-11]; positivity for ANA in high titer or the presence of anti-Ro/SSA or anti-La/SSB antibodies were diagnostic criteria for Sjögren’s syndrome [10]; the presence of anti– Jo-1 antibodies was a criterion for the diagnosis of dermatopolymyositis [12,13]; the presence of anticentromere or anti–topoisomerase I (antiScl70) antibodies was the criterion for classifying subtypes of cutaneous systemic sclerosis as limited or diffuse [9].
The multiplex immunodot assay: We used for the diagnosis of autoimmune diseases the following Immunodot kit (biomedicals diagnostics, France)
Screening for HIV, HBV and HCV: Chimiluminescent microparticle immunoassay (CMIA) technology (Architect ci4100®) was used in screening for HIV, HBV and HCV.
Screening for CMV and Lyme diseases: Enzyme linked Fluorescence Assay (ELFA) technology (MiniVidas®) was used in screening for CMV and Lyme diseases. Dengue serotype 1,2,3,4,5 and Mycobacterium tuberculosis were determined using the immunochromatographic technical (Hexagon D® and Hexagon TB®° respectively).
Data were analyzed using Wilcoxon test to compare rates. We used Fischer (t) test for the percentages. Non parametric tests (Kruskal Wallis and Mann-Witney) were used when normality of distribution wasn’t verified. Results were considered statistically significant when p<0.05. Analysis was performed with STATA™ .
The number of visits during the study period was 73950 including 17 cases of autoimmune diseases. The referral diagnosis was right in 17/65 patients (26.2%). The incidence was 17/73950 (0.023%).
Table 1 shows demographic and clinical characteristics of autoimmune diseases patients in the study cohort. Comparing gender impact on the autoimmune diseases, the women were more likely to have autoimmune diseases than men (p=0.004). Analyzing correlation between autoimmune diseases and race or ethnicity and family annual incomes, no differences attributable to ethnicity and family annual incomes were observed (p=0.7).

Table 1: Demographic and clinical characteristics of patients with in the study cohort (n = 65)
Antibodies against HBV or HCV (Hepatitis B or C virus), HIV, CMV, Borrelia burgdorferi were found in sera or saliva of patients with autoimmune diseases such as SSG, SLE, and PR. No correlation was found in our study (p=0.3).
The table 2 show inflammatory proteins profiles in the sera of patients with autoimmune diseases in the study cohort. Analyzing the different fractions of proteins, it’s found that the hypoalbumininemia is associated with autoimmune diseases (p=0.02). Thus hypergammaglobulinemia is associated with SLE and hypogammaglobulinemia is associated with Hashimoto Thyroiditis.
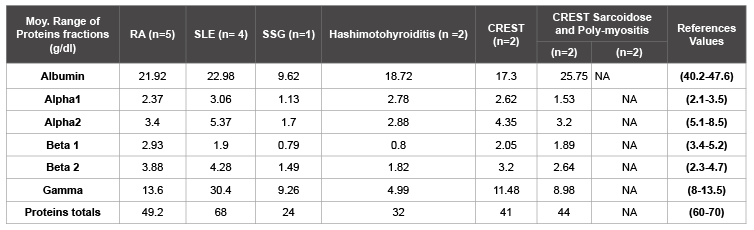
Table 2: Inflammatory protein profile in the sera of patients with autoimmune diseases
NA: Not Applicable
The table 3 show the auto antibodies profiles in the sera of patients with autoimmune diseases in the study cohort. Comparing the proportion by type of autoimmune disease, it is found that there is no significant statistical difference in ours cohort (p=0.07).
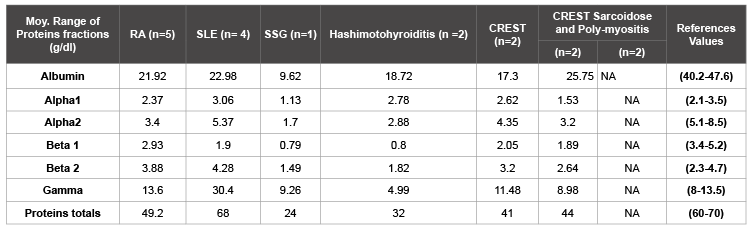
Table 3: Auto antibodies profiles in the sera of patients with autoimmune diseases
In ours study, there was a predominance of women among the affected member of study. Overall, 94.12% (16/17) of affected individuals were female and 5.88% (1/17) of affected individuals were men. Performances and the diagnosis of Immunodot technique were respectively 100% and 57% for the sensitivity and specificity.
The correct use and interpretation of serologic testing for diagnosing autoimmune diseases present a challenge to clinicians for two reasons: first the sensitivity and specificity of most laboratory tests for autoimmune disease are significantly less than 100% and second the detection of autoantibodies using different techniques such as indirect immune fluorescence or multiplex Immunodot assays give different results.
The table 4 show the proportion of autoimmune diseases and patient’s socioeconomic status in ours study. Comparing pregnancy’s impact on autoimmune disease occurrence, it is found that women who have had multiples pregnancies are more affected than primiparous (p=0.002).
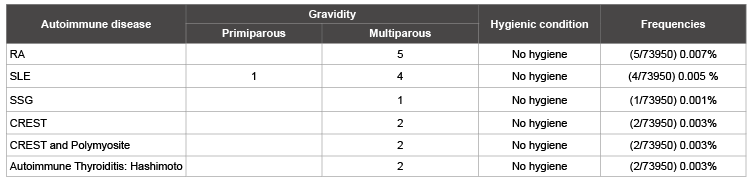
Table 4: Rate of autoimmune diseases and patient’s socio economic statute
The figure 1 show the Women’s age and autoimmune diseases.
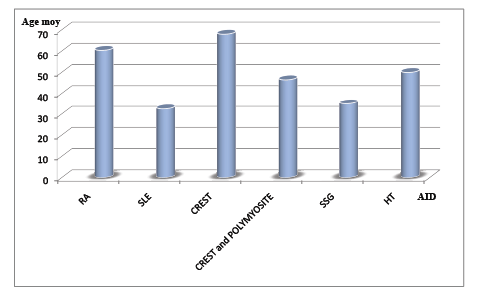
Figure 1: Women’s age and autoimmune diseases
Comparing different age proportions depending on the autoimmune disease, SLE and SSG were more frequently in young women old of 32.5 years and 35 years respectively (Khi 2=5.30 ). The old women were more likely to have CREST (68 years), RA (60.4 years), with HT (50 years) and CREST and Polymyositis (46.5 years) (Khi 2 = 3.72).
In this study, the incidence of autoimmune diseases is very low (0.023%) and all except one patient were women. The referral diagnosis was right in 17/65 patients (26.2%) among patients in the limited setting country (Burkina Faso). Data analysis confirmed the low frequency of autoimmune diseases. There were 0.005% patients with systemic lupus erythematosus (SLE), 0.007% with rheumatoid arthritis (RA), 0.003% with CREST and Polymyositis, 0.003% with CREST, 0.001% with Gougerot Sjögren’s syndrome (SSG), 0.003% with Thyroiditis of Hashimoto and, 0.001% with sarcoidosis. Autoimmune diseases prevalence is in the range of 4-10% in American and European populations [8,14-18]. Given that the prevalence of autoimmune diseases in USA and Europe is 4-10%, as referred, the 0.023% of autoimmune diseases found is very low and it probably simply indicates that there are many other patients with autoimmune diseases non-diagnosed. We report that SLE and SSG were more frequently in young women old of 32.5 years and 35 years respectively. The old women were more likely to have CREST (68 years), RA (60.4 years), HT (50 years) and CREST and Polymyositis (46.5 years). This gender inequality found in autoimmune diseases is often due to hormonal factors [3-8,19,20]; antibodies production and the presence of lymphocytes are higher in women than men [2]. Indeed, in the mouse model of MS, experimental autoimmune encephalomyelitis in mice, disease severity is increased by castration in male mice diffuse and decreased by testosterone implantation in females diffuse [4].
The immunomodulatory effects of gonadal steroids, especially, testosterone, may underlie the female-specific association with autoimmune disorders.
We additionally report positive association with RA, SSG, HT, CREST/ Polymyositis and gravidity. These results were reported in many studies [12,13,21-24]. It suggests that transition from fetal cells through the placenta during pregnancy, and their persistence in the mother after childbirth can in some cases disturb immunity which explains that the women multiparous are more likely to have autoimmune diseases than those with one pregnancy.
In our study we have found that there is no association with hygiene hypothesis and autoimmune disease. The annual incomes was very low in our study (< 5760$ USA). Studies that have investigated human autoimmune disease risk in relation to the hygienic degree have found many associations [24].
We also examined a negative association with RA, SLE, CREST/ Polymyositis, HT and infection agents (HIV, HBV, HCV, Lyme disease, Dengue serotype 1, 2, 3 and 4) a positive association with Mycobacterium tuberculosis and RA cases (3.07%).
It is a possibility that autoimmune disease and the associated Mycobacterium tuberculosis infected may modulate the immune response and RA risk, given some observed correlation between the Mycobacterium tuberculosis and autoimmune diseases [1,2,9-13]. It would be of interest to evaluate RA risk in relation to the Mycobacterium tuberculosis infection in populations where Mycobacterium tuberculosis frequencies are sufficiently high (e.g., Africa tropical); and perhaps more importantly, in any population stratified by gender.
Our findings are, however, consistent with a direct identification of autoantibodies in patients samples coupled with clinical signs. Identification of autoantibodies is essential for the diagnosis of autoimmune diseases in limiting setting country. Clinicians can use these results as guidance for classifying patients and/or for assessing their response to specific therapies, especially in cases where development of targeted biological therapies [12,13,21].
Ours technologies have provided a new approach for autoantibody quantification based on multiplex testing which represents an advantage compared to earlier methods such as line blot assays and conventional one well-one test microtiter ELISA diffuse [3,22,23]. These multiplex assays have made it possible to simultaneously detect multiple biomarkers using a single platform and a single serum sample. In this study we have showed different levels of clinical sensitivity for the detection of the most frequently detected autoantibodies in AID patients.
BMD Test® allows testing of seven antigens in parallel from individual serum samples with high specificity (99.8%). This method provides significant savings in time, avoiding the conventional first screening and post-confirmation algorithm currently being used in most clinical testing laboratories for AID sample testing. BMD requires only 20μL of sample and follows the same steps as a traditional ELISA assay. Together with low setup costs and a fast sample processing time, BMD provides an affordable alternative to currently available multiplex testing systems. These cost-saving features enable small laboratories with limited budgets to use this technology without a large capital outlay and training of laboratory technicians. BMD have demonstrated an excellent analytical sensitivity (100%) but a bad specificity (57%) and when compared with established ELISA assays [4,8,11]. Moreover, when compared with a line immunoassay, BMD represents the quantitative advantage for clinical management of patients [25-28].
The referral diagnosis was right in 17/65 (26.2%). The incidence was 17/73950 (0.023%) in the limited setting country (Burkina Faso). The very low incidence of auto-immune diseases found is probably due to a number of undiagnosed patients. There was not a significant association between auto-immune diseases and infection; there was not also significant association between auto-immune diseases and hygienic hypothesis.
We thank investigators at University Hospital, Bobo Dioulasso BURKINA FASO Dr. (University Hospital) for providing patients with autoimmune diseases and clinical data.
Conceived and designed the experiments: Y Sourabié, Y Traoré, F Fumoux, M S Ouédraogo, and C Sirima
Performed the experiments: Y Sourabié, Y Traoré and M S Ouédraogo
Analyzed the data: Y Sourabié and Y Traoré
Contributed reagents/materials/analysis tools: Y Sourabié, Y Traoré and F Fumoux,
Wrote the paper: Y Sourabié, Y Traoré, F Fumoux, M S Ouédraogo, and C Sirima
We declare that we have no conflict of interest.
Download Provisional PDF Here
Article Type: Research Article
Citation: Sourabié Y, Bazié WW, I Sangaré, Sirima C, Ouédraogo MS, et al. (2016) Autoimmune Diseases in Limited Setting Country (Burkina Faso): Epidemiology, Technical and Diagnostic Performance of Specific Biomarkers for the Early Diagnosis. Autoimmun Infec Dis 2(2): doi http:// dx.doi.org/10.16966/2470-1025.112
Copyright: © 2016 Sourabié Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access