Introduction
One of the goals of using advanced reproductive technologies is to increase the reproduction rate of breeding animals of high genetic value. In alpacas, the use of reproductive biotechnology techniques has been minimal. These are probably due to the complex reproductive characteristics of alpacas at the anatomical and physiological level more recently demonstrated by molecular biology [1] and on the other hand, to the lack of research support [2]. However, in recent decades more attention has been giving to basic and applied studies of these technologies due to the higher recognition of the market for the excellent quality of alpaca fiber, which generates higher economic returns for producers and in turn, it has been the main driver for Research and Development (R&D) programs [3].
Alpacas and South American camelids, in general, have a low reproductive efficiency, about 50% of the females do not produce offspring within a year, 50% of embryos die in the first month of gestation [4] and 20% of females fail to become pregnant after mating [1]. Therefore, a more complete understanding of folliculogenesis, oogenesis, estrous, ovulation induction, formation and regression of the Corpus Luteum (CL) that can lead to improvement of the quality and viability of oocytes is needed [1]. Currently in alpacas, obtaining embryos in vivo by super ovulation and uterine flushing has an average yield of 4.75 embryos per female [5]. The first successful In vitro embryo production in alpacas was reported the yield of 8% transferable embryos (at morula and blastocyst stage) from slaughterhouse oocytes In vitro matured and fertilized with frozen sperm obtained from the epididymis [6]. A recent study shows 29.7 ± 3.8% blastocysts rate [7] which is a significant progress but still the In vitro embryo production technology in alpacas requires further improvements compared to other domestic species. One of the factors limiting the development of the In vitro embryo production technology in alpacas is the low availability of oocytes due to the small number of sacrificed females and the unique reproductive characteristics that do not allow the embryo culture systems to be directly extrapolated from other species [8] being necessary to develop specific protocols for maturation, fertilization and embryonic development In vitro for alpacas.
Oocyte maturation is the most critical process in In vitro culture, since the oocyte acquires competence for future processes, which involve the resumption of meiosis, epigenetic regulation, polyspermia block, activation of the zygote, exchange of protamines to histones, cleavage, cell polarization, embryonic stem cell proliferation and implantation [9]. Oocytes can be collected from females with or without hormonal therapy for multiple follicular developments, by ultrasound-guided transvaginal follicular aspiration known as “Ovum Pick Up” (OPU), follicular aspiration of ovaries exposed by laparotomy, follicular aspiration through laparoscopy, or by follicular aspiration of ovaries obtained from euthanized animals. The latter method is where a greater number of cumulus-oocyte complexes are obtained using sequential aspiration and dissection of the ovary [10]. However, heterologous populations of preantral and antral follicles are obtained and those that could have suffered atresia and irreversible apoptotic changes that will have repercussions and produce a decrease in the maturation rate In vitro [11]. For the oocytes to achieve maturation, all the molecular machinery must be regulated in space and time, thus achieving the transition from germinal vesicle to metaphase II, in addition to the production and storage of transcripts for use in fertilization and first divisions of the embryo until activation of zygotic genes [12]. The events that lead to obtaining a quality mature oocyte are given by a complex and dynamic interaction between the antral follicle and the oocyte where somatic cells (theca, granulosa and cumulus), basal lamina and Follicular Fluid (FF) participate. During this process, the oocyte must complete its final growth, and nuclear and cytoplasmic maturation [13]. In vitro maturation in alpacas is commonly carried out by extrapolating culture medium from cattle to alpacas, generating highly varied results [14].
The application of advanced reproductive technologies such as In vitro maturation, In vitro fertilization, intracytoplasmic sperm injection, In vitro embryo culture, cloning and transgenics require quality oocytes. Currently, there are morphological predictors for the quality of the COCs (Cumulus Oocyte Complexes) such as the compactness and thickness of the cumulus investment and brightness of the ooplasm; the granularity, coloration, inclusion and regions of organelle clustering of the cytoplasm; the shape of the polar body (round vs. Ovoid); its size (large or not); surface (smooth vs. rough); intactness (intact vs. fragmented), the thickness and organization of the zona pellucida, the size (enlarged or not) and the presence or absence of particles, of the perivitelline space; the structures and characteristics of the follicle/ovary (Follicle dimension; existence of CL with a follicle dominant or a cyst), and cellular/molecular predictors such as mitochondria (Mitochondrial distribution, Adenosine Triphosphate (ATP) and Mitochondrial DNA (mtDNA) levels), glucose 6-phosphate dehydrogenase (G6PDH) (G6PDH activity (BCB+ or BCB-)), calcium (Plasma membrane calcium current in the immature oocyte, Calcium stores in mature oocyte), phosphodiesterase 3 (Intracellular phosphodiesterase 3 activity), glutathione (Intracellular glutathione level), apoptosis (Apoptosis in granulosa or in cumulus cells, Ratio of B-cell Lymphoma gene 2 (Bc l-2) to Bcl-2-Associated X protein (Bax)), Insulin-like Growth Factor (IGF) and Insulin-like Growth Factor Binding Protein (IGFBP) (Concentrations of IGF-1 and IGFBP-1 in FF; IGF-I/IGFBP-1 ratio in serum or FF; expression profile of lowmolecular-weight IGFBPs (IGFBP4 and IGFBP5) in FF), super family of transforming growth factor beta (Inhibin levels in FF; activin A level in FF; basal levels of anti-Müllerian hormone in serum), steroids (Ratio of oestradiol/testosterone or oestradiol/progesterone) in FF; level of oestradiol, LH, progesterone or prolactin in FF), oxidative stress (Quantity of 8-hydroxy-2’-deoxyguanosine (8-OHdG) in granulosa cells; level of nitric oxide, lipid peroxides, total anti-oxidant capacity and reactive oxygen species (ROS) in FF), leptin (Leptin levels in FF or serum) and gene expression profiles (Expression of hyaluronic acid synthase 2, cyclo-oxygenase 2 and gremlin 1 in cumulus cells; relative abundance of pentraxin 3 in cumulus) [15]. In alpacas, few predictors of oocyte quality have been used, more research is required. Therefore, the objective of this study was to evaluate the selection of competent alpaca oocytes matured In vitro in two different culture media by morphometry, cytoplasmatic, and nuclear staining
Materials and Methods
Chemical reagents and statement of ethics
All reagents used are from Sigma-Aldrich (Burlington, USA) unless otherwise noted. All prepared media filtered prior to use (0.22 μm; Aijiren, China). All the experimental procedures were carried out in accordance with the Animal Ethics Committee of the Universidad Nacional del Altiplano, complying with all the ethical principles of animal research.
Location and experimental animals
The experiment was carried out in the Puno region (altitude: 3 847 meters above sea level, Latitude: 15º 50’26”South, Longitude: 70º 1’ 41” West). A total of 22 Alpacas (Vicugna pacos) were used, with a body condition score of 3.28 ± 0.52 (scale of 1-5) with ages between 3-5 years. Nuclear and cytoplasmic maturation analyzes performed at the Animal Reproduction Laboratory of the Universidad Nacional del Altiplano, Puno, Peru.
Slaughter-derived oocytes
The alpaca ovaries (n=44) collected from the Nuñoa slaughterhouse, Puno, within 30 minutes after the sacrifice and then transported for 6 hours to the laboratory in thermoses at 33-36ºC in a saline solution supplemented with streptomycin (0.05 mg/mL). At laboratory follicles larger than 3 mm aspirated with a 20G x 1-inch needle attached to a 5 mL syringe and follicles smaller than 3 mm were dissected.
Maturation culture medium
The maturation medium 1 (MM1) prepared based on media reported by Tribulo [16]. MM1 contains TCM-199, 10% (v/v) fetal calf serum, 2 μg/mL 17β-estradiol, 25 μg/mL porcine Follicle-Stimulating Hormone (pFSH) (Folltropin®-V; Vetoquinol, Canada), 22 μg/mL sodium pyruvate, 50 μg/mL of gentamicin sulfate and 1mM of alanylglutamine. The maturation medium 2 (MM2) prepared based on what was reported by Saadeldin [17]. The MM2 contains TCM-199, 25 mM HEPES-buffer, 2 mM NaHCO3, 10% (v/v) of fetal calf serum, 10 μg/mL pFSH, 10 μg/mL Human Corionic Gonadotropin (hCG) (Choriomon; IBSA, Switzerland), 1 μg/mL 17β-estradiol, 20 ng/mL Epidemic growth factor (EGF), 1 μg/mL insulin, 0.3 μM cysteamine, 1 mM L-glutamine, 50 μg/mL gentamicin sulfate.
Experimental design
The cumulus-oocyte complexes (COC) were randomly distributed in the two experimental groups (MM1 and MM2) with three replicates per experiment. After In vitro maturation, the oocytes stripped with 1% hyaluronidase for 15 minutes at 28.5ºC under conditions of 5% CO2 . Subsequently, the oocytes are distributed for nuclear and cytoplasmic maturation analyzes, as well as morphological analyzes.
Oocyte maturation
The COCs selected using a stereomicroscope and the oocytes that had more than one layer of cumulus cells were selected. The 212 selected COCs assigned to each of the two experimental groups (MM1 and MM2). Every 20-30 oocytes transferred into wells with 60 μL of maturation medium, covered with mineral oil and cultured in a portable incubator for 40 hours [18] under conditions of 5% CO2 (produced by effervescent granules) and 38.5ºC (using a thermostatic bath) [19].
Morphometry of the oocytes
The oocyte diameters, thickness of the zona pellucida and area of the perivitelline space of the experimental groups (MM1 and MM2) were compared. Photographs taken with a Leica inverted microscope camera. The mean determined and measurements were made with the ImageJ software (version 1.8.0; National Institutes of Health) (Figure 1).
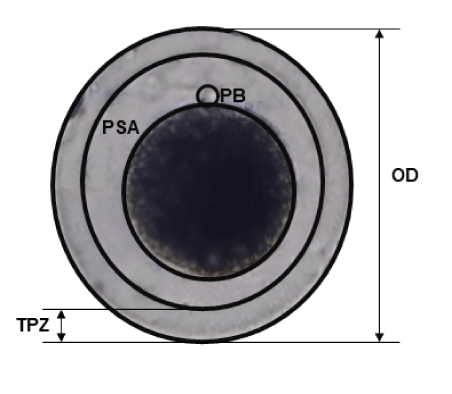
Figure 1: Morphometric analysis of Alpaca in vitro matured oocytes. Captured images were analyzed by using ImageJ software to measure the pixel distance. OD: Oocyte Diameter; TPZ: Thickness of Zona Pellucida; PSA: Perivitelline Space Area; PB: Polar Body.
Assessment of nuclear maturation
The recovered COCs exposed to a 1% hyaluronidase solution for 15 minutes at 38.5ºC in 5% CO2 and subjected to successive pipetting to separate the cells from the oocyte cluster. Then, the oocytes fixed with a solution of ethanol: glacial acetic acid (3:1) in an airtight environment for at least 48 hours at 4ºC [20]. The fixed oocytes were placed from 5 to 10 oocytes between the slide and the coverslip, and a mixture of vaseline: paraffin (2: 1) placed in each corner of the coverslip. The coverslip was lightly pressed until contact made with the oocytes. The staining was carried out by capillary action with a solution of 1% orcein in 45% acetic acid for 10 minutes. The assessment of nuclear maturation performed using a Leica inverted microscope (Leica Microsystems; Wetzlar, Germany; 40X). The oocytes are classified based on the presence of the Germinal Vesicle (VG), metaphase I (MI), metaphase II (MII) and degenerated structures (DG). Degenerated oocytes presented chromosomal aberrations, presence of diffuse or undefined chromatin (Figure 2).
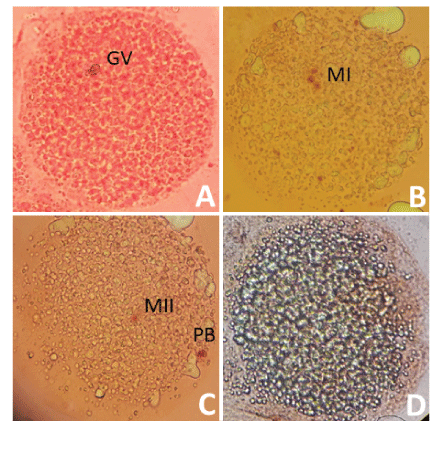
Figure 2: Classification of nuclear maturation in Alpaca oocytes.
Nuclear maturation assessed using acetate-orcein staining. A) Oocyte in the germinal vesicle stage, B) Oocyte in state of metaphase I, C) oocyte in state of metaphase II and D) Degenerated oocyte. GV: Germinal Vesicle; MI: Metaphase I; MII: Metaphase II; PB: Polar Body.
Assessment of cytoplasmic maturation
Denuded oocytes incubated in a 26 μM brilliant cresyl blue (BCB) solution (Toronto Research Chemicals INC; Toronto, Canada) in phosphate buffered saline (PBS) for 90 minutes at 38.5ºC in 5% CO2 [21]. Assessment of cytoplasmic maturation performed using a Leica inverted microscope. Oocytes classified according to the presence (BCB +) or absence (BCB -) of BCB staining (Figure 3).
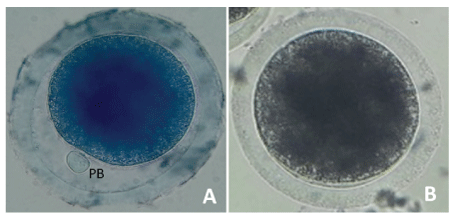
Figure 3: Classification of cytoplasmic maturation in Alpaca oocytes.
Cytoplasmic maturation using BCB staining. Oocytes are classified according to the presence (A) and absence (B) of BCB staining. BCB: Brilliant Cresyl Blue; PB: Polar Body.
Statistical analysis
All statistical analyzes and graphs performed using the data analysis system for Windows (GraphPad Prism version 8.0.1 software and the Microsoft Excel data analysis tool). Student’s t-test analysis performed for oocyte diameter, zona pellucida thickness, perivitelline space area, nuclear maturation, and cytoplasmic maturation for the two experimental groups. The results are presented as means ± standard deviation. A probability level of P <0.05 is considered statistically significant.
Results
Effect of the two maturation culture media on oocyte morphometry
The diameter of the oocytes after In vitro maturation (Figure 4) were 133.42 ± 14.56 μm and 136.14 ± 15.24 μm for the MM1 and MM2 respectively. There were no significant differences (P=0.397) between the groups. On the other hand, no significant differences were observed (P=0.919) with respect to the thickness of the zona pellucida (Figure 5) in the MM1 (16.84 ± 3.96) μm and MM2 (16.73 ± 4.98) μm culture medium, but there were significant differences (P=0.0005) between the area of the perivitelline space (Figure 6) of the MM1 (11018.91 ± 2465.40) μm2 and MM2 (8686.18 ± 3016.88) μm2.
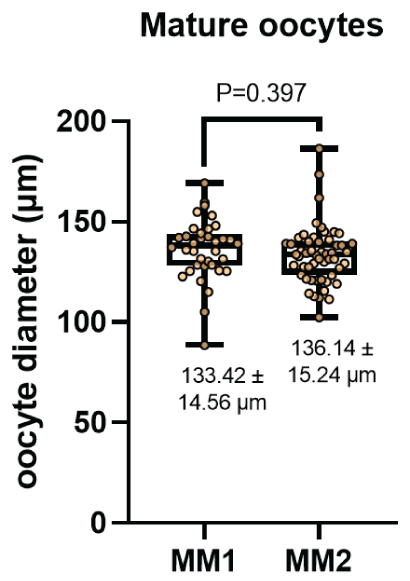
Figure 4: Oocyte diameter measurement (μm) after in vitro maturation using two maturation media; MM1 and MM2. No significant differences (P=0.397) were found between the two experimental groups.
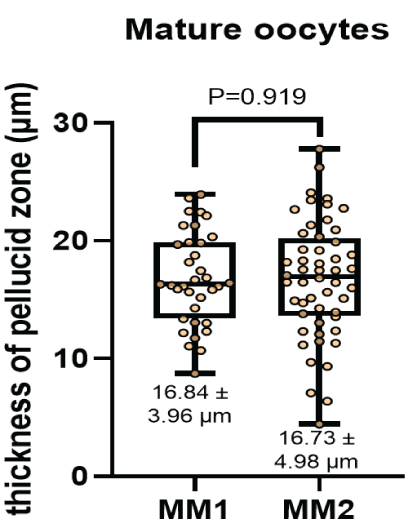
Figure 5: Thickness of zona pellucid measurement (μm) after in vitro maturation using two maturation media; MM1 and MM2. No significant differences (P = 0.919) were found between the two experimental groups.
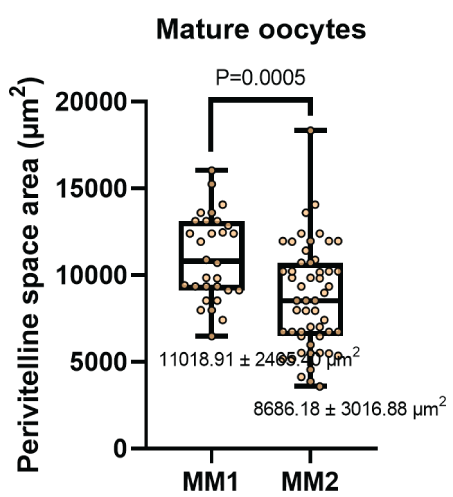
Figure 6: Perivitelline space area measurement (μm) after in vitro maturation using two maturation media; MM1 and MM2. Significant differences (P=0.0005) were found between the two experimental groups.
Effect of the two maturation culture media on oocyte nuclear maturation
Figure 7 shows the percentage of nuclear maturation of oocytes after In vitro maturation [22]. The metaphase II percentages were 46.08 ± 5.90% and 54.65 ± 3.0% for the MM1 and MM2 respectively. There were no significant differences (P=0.088) between the two culture mediums. The same was observed for the percentage of metaphase I (31.54% and 20.08%) germinal vesicle (8.51% and 9.01%) and degenerated structures (13.87% and 16.26%) for MM1 and MM2 respectively.
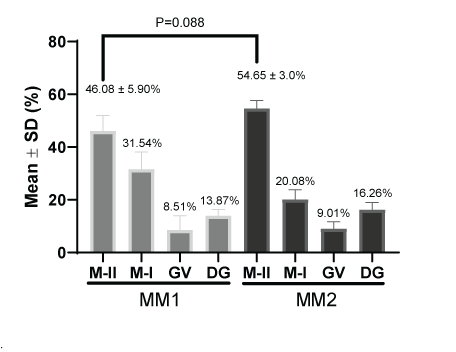
Figure 7: Nuclear maturation measurement (%) after in vitro maturation using two maturation media; MM1 and MM2. No significant differences (P=0.088) were found in metaphase II between the two experimental groups. MII: Metaphase II; MI: Metaphase I; GV: Germinal Vesicle; DG: Degenerate.
Effect of the two maturation culture media on oocyte cytoplasmic maturation
Figure 8 shows the percentage of BCB + staining for cytoplasmic oocyte maturation after In vitro maturation. There were significant differences (P=0.014) between culture media in the percent of cytoplasmic maturation; oocytes cultured in MM1 (67.78 ± 4.50 %) had a lower cytoplasmic maturation compared to MM2 (77.61 ± 3.58%).
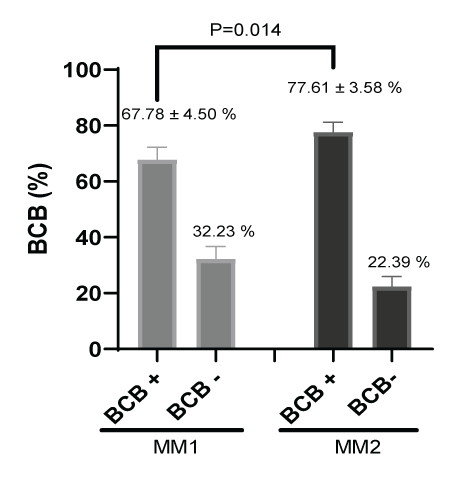
Figure 8: Cytoplasmic maturation measurement (%) after in vitro maturation using two maturation media; MM1 and MM2. Significant differences (P=0.014) were found between the two experimental groups. BCB: Brilliant Cresyl Blue.
Discussion
The results of the present study are the first to describe determination of oocyte competence according to the nuclear, cytoplasmic and morphometric changes in alpaca oocytes during In vitro maturation comparing two different culture media. The In vitro maturation period was 40 hours for the two experimental groups. This value was taken according to previous work on In vitro maturation in alpacas [3,23,24] and camels [25,26], which resulted in high rates of metaphase II (MII) oocytes. Furthermore, it is closely related to high rates of blastocyst formation after In vitro fertilization. The use of longer periods generates a higher rate of degenerated oocytes. The recovery of oocytes is of great importance to carry out advanced studies in reproductive biotechnology. There are few slaughterhouses in the world where alpacas are slaughtered, being the Puno region where there is the largest population of alpacas in Peru, and the district of Nuñoa the place where the greatest quantity is slaughtered. However, limited animal material is available for laboratory experimentation. It is for this reason that two sequential oocyte collection procedures were used to obtain the highest possible performance, which first consists of the aspiration of follicles using 5 mL syringes larger than 3 mm and then dissection of the ovaries to obtain follicles smaller than 3 mm. The aspiration using only a syringe or vacuum pump (commonly applied in other domestic species) [27], shows a low recovery rate in alpacas. This is mainly attributed to the ovary structure of the alpacas and camels [25].
The incubators used for In vitro culture recreate the in vivo conditions in the process of maturation, fertilization and embryonic development. Different technological strategies have been designed that vary depending on the rate of embryos obtained and the economic factor [28]. In previous studies carried out in our laboratory, it was shown that there are no significant differences between a conventional incubator and a portable one in obtaining bovine embryos [19]. The latter uses effervescent granules as a supply of CO2 (5%) and a thermostatic bath as a temperature regulator (38.5ºC). Additionally, in highland conditions, fluctuations in ambient temperature vary from -7 to 18ºC [29]. These extreme temperature changes make the use of conventional incubators less viable than incubators with a thermostatic bath. Since the latter response much better to sudden changes in ambient temperature.
In our investigation to determine oocyte competence after in vitro maturation according to nuclear, cytoplasmic maturation and morphometric, two culture media were used (MM1 [16] and MM2 [17]). In most studies carried out in alpacas, MM1 is generally used for maturation; however, until now it was not known whether it was the most efficient maturation medium. According to our results, MM2 has a higher yield than MM1 both in nuclear maturation (46.08 ± 5.90% for MM1 and 54.65 ± 3.0% for MM2) and in cytoplasmic maturation (67.78 ± 4.50 for MM1 and 77.61 ± 3.58 % for MM2), although only significant differences were found in cytoplasmic maturation. On the other hand, our results are within the range reported by other researchers. In alpacas, nuclear maturation of 65.8 ± 8.1% at 38 h [30], 65.1 ± 5.7% at 32 h by syringe aspiration [7], 75.3 ± 11.9% at 42 h [18], 57.6% using follicles larger than 2 mm, supplemented with fetal bovine serum 10 %(v/v) are reported and animals older than 11 years [14], 64.9% ± 8.1%, in the presence of 0.5 μg/mL FSH for 42 h [23], 46.9% using 0.25 μg/mL, 2.5 μg/mL and 15 IU/mL [31], 74.2%, 2 IU/mL of eCG and 10 IU/mL of hCG [32]. In llamas, 70.53 ± 3.75%, 42 hours [33]. In dromedary camels, 81.6 ± 0.8% supplemented with FCS 10% (v/v) at 30-32 hours [34], 61% using TCM-199 medium, cultured at 40-42 h, slaughterhouse oocytes and using the dissection method [25], 71% at 42 h [35]. In alpacas, cytoplasmic maturation studies have not yet been reported, being the first in this study. However, in cattle, rates of 80.3% [36] and 77.75% have been obtained by aspirating follicles from 4 to 8 mm using OPU [21]. Furthermore, in this same study, no significant differences were found in animals with and without super ovulation treatment.
According to our morphometric studies performed on oocytes matured with two maturation media, for the case of oocyte diameter 136.14 ± 15.24 μm and 133.42 ± 14.56 μm, thickness of zona pellucida 16.73 ± 4.98 μm and 16.84 ± 3.96 μm, area of the perivitelline space 8686.18 ± 3016.88 μm2 and 11018.91 ± 2465.40 μm2 using MM2 and MM1 respectively. No reference works were found in alpacas. However, in dromedary camelids there is a morphometric study with oocyte diameter 145.93 ± 0.85 μm and 141 ± 0.87 μm, the thickness of zona pellucida 10.52 ± 0.31 μm and 10.76 ± 0.38 μm, for oocytes with first polar and degenerated bodies respectively [26]. Comparing these studies shows that oocytes with a larger diameter and a thinner zona pellucida are related to quality oocytes and a high fertilization rate [37], obtaining better results with the MM2. In the case of the area of the perivitelline space, it is known that a greater area is related to a low fertilization rate [38], having better oocyte quality results using MM2.
The differences between the culture media are due to their composition and concentration of the reagents used in each of the culture media. Regarding the concentration of pFSH, there are 25 μg/ ml compared to 10 μg/ml, 17β-estradiol of 2 μg/ml against 1 μg/ml for MM1 and MM2 respectively. However, MM1 does not contain hCG or EGF in its composition, these molecules being crucial to obtain competent oocytes from alpacas. Since, hCG participates in the molecular mechanism of maturation and EGF participates in the molecular mechanism of oocyte growth. On the other hand, the administration of cysteamine and insulin in MM2 helps in cellular deinoxification and in the energy metabolism of the oocyte. We recommend conducting more studies using these reagents individually and collectively, as well as, supplementing with cytokines such as fibroblast growth factor, insulin-like growth factor, leukemia inhibitory factor, among others. At the same time to carry out studies of the molecular mechanisms that sublimate the signaling of the aforementioned molecules. Likewise, it is recommended to carry out complementary studies with predictors of oocyte quality at the molecular level.
Conclusion
The present study shows that MM2 presents better results in the cytoplasmic maturation and area of the perivitelline space. However, nuclear maturation, the diameter of the oocyte and thickness of the zona pellucid did not show significant differences between the culture media. This is due to the fact that MM2 contains epidermal growth factor, human chorionic gonadotropin, cysteamine and insulin in its composition that participate directly and indirectly in obtaining competent oocytes from alpacas.
Acknowledgments
The authors thank the District Municipality of Nuñoa for facilitating the entrance to the slaughterhouse in their jurisdiction. Likewise, to INIA-Donoso and Vivanco International S.A.C. for the training provided. This study was financed by the National Council of Science, Technology and Innovation (Concytec), which is an entity attached to the Presidency of the Council of Ministers in Peru.
Author Contributions
DHM: Conceptualization, supervision, methodology and data curation; performed experiments, statistical analysis, interpretation of data, writing original draft, reviewing and editing the final version. FWG: Conceptualization, supervision, methodology and data curation; performed experiments, final version review. MGP: Conceptualization, supervision, methodology and data curation; performed experiments and final version review. BDV: Conceptualization, supervision, methodology and data curation, final version review. HWV: Conceptualization, supervision, methodology and data curation; performed experiments and final version review.
Declaration of Competing Interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.









