Abstract
The current study examined the effect of smoking habits on the presence of candida in the oral cavity. The study group included 50 healthy smokers (age range 20-50 years, mean 26.78 years, median 25 years and SD ± 6.9). As reference we examined a group of 50 healthy nonsmokers with similar form of age and gender. This study was the first to use the Diaslide device, which was modified to provide a quantitative assessment of candidal colonies.
Of all the parameters which evaluated the influence of smoking on candida, the number of colonies in saliva was the only one that was significantly higher in smokers compared to non-smokers (p=0.037).
The current study had demonstrated that smoking appears to have a minor influence on candidal colony growth, compared to other known factors such as dry mouth, immunodeficiency disorders or antibiotic treatment. It is recommended that the study will be repeated with participants of different combinations of age and smoking habits, and possibly research differences between varied candida species in association with tobacco smoking in the oral cavity.
Keywords
Candida; Tobacco smoking; Diaslide
Introduction
Candida albicans is the most common fungal species isolated from the
oral cavity [1] and is easily cultured from the posterior dorsum of the
tongue. This dimorphic fungus may present in two phases: the blastospore
phase and hyphal phase. The later can penetrate deeper into the tissue
especially when the host defense mechanism is compromised [1].
A number of studies have found that smoking either alone or in
combination with other factors may be an important predisposing factor
for oral candidiasis, although this relationship or its pathogenic influence
on oral Candida is far from being resolved [2]. While some studies have
suggested that smoking does not affect Candida carriage significantly
[3-8], other have reported that smoking significantly increases its’
prevalence [9-11]. Cigarette smoking seems to have a contributing
effect especially on the incidence of pseudomembranous candidiasis in
immunocompromised individuals [12].
The exact mechanism by which candidal carriage may be affected by
cigarette or cigar smoke is not yet established [2]. It has been suggested that
cigarette smoking might lead to localized epithelial alterations allowing
candidal colonization [9]. Cigarette smoke may also provide nutrition
for candida albicans [13]. This assumption has important implications
as the aromatic hydrocarbons contained in cigarette smoke may also be
converted, by inducible enzyme systems present in candida species, into
carcinogen end products [14,15]. These theories offer partial explanations
why smokers may be more prone to candidal leukoplakia with higher
potential for malignant changes than other leukoplakias [16,17].
The Diaslide device is a clinical tool for identification of urine bacterial
colonies [18]. The current study was the first study that used a Diaslide
device which was modified by substitution of the growing media of the
original Diaslide (MacConkey agar) to the Agar Sabouraud, a candida
selective media [19], containing chloramphenicol to suppress the presence
of bacteriae. In the present study it was used as a clinical tool to provide a
quantitative assessment of candidal colonies (Figure 1).
The main purpose of this study was to validate the association between
smoking and the quantitative assessment of candida colonies from the
oral cavity using a modified Diaslide as a quantitative clinical tool.
Materials and Methods
The study group consisted of 50 individuals (25 men, 25 women)
healthy smokers at the ages of 20-50 years (mean 26.78). The matched
control group included 50 individuals (27 men, 23 women) healthy
nonsmokers, at the ages of 20-50 years (mean 25.94).
The term “Smoker” defined a participant who smoked over 10 cigarettes
a day for at least three years. Parameter of “pack years” was calculated
(cigarettes per day multiplied by smoking years).
The term “Nonsmoker” defined a participant who had never smoked or
didn’t smoke for minimum five years.
The term “Healthy” defined a participant who was free of any
compromised medical condition did not receive any treatment known
as promoting oral candidiasis (antibiotics, steroids, high blood pressure
medication, anemia due to iron deficiency, diabetes, AIDS etc.).
Samples for candidal culture were obtained from the back of the tongue
and from the saliva. In order to minimize the effect on the presence of
Candida in the oral cavity, samples were taken at least two hours after
eating, drinking and any oral hygiene procedure.
Sampling methods: the quantitative assessment of candida tool
included the modified Diaslide device. The original device referred to
bacterial colonies from urine [18].
The modified device platform was Sabouraud dextrose agar which is
Candida selective, containing chloramphenicol to suppress the presence of
bacteria. Antibiotics, stains and some salts have been added to Sabouraud
medium for bacterial inhibition and as yeast indicators [19].
The device consists of a hinged case containing two opposing agar
Sabouraud media separated by a sampler with a handle at one end and
two bent sampler tips at the opposite end. The tips are first dipped into the
sample. The sampler is then pulled out through the casing, simultaneously
inoculating both agar surfaces.
Candida albicans inoculation was primarily performed in order to
introduce a reference chart characteristic of candida colonies. Instrument
calibration was performed based on known concentrations of Candida
(CFU/mL) in order to enable the comparison of different cultures (Figure
1). Based on these calibrations the number of colonies obtained on
the diaslide could be translated into a numerical quantitative extent of
candidal growth.
- The following results were obtained (CFU/mL);
- 102/ml - One Colony
- 103/ml - two colonies
- 104/ml - Twenty colonies
- 105/ml - above twenty colonies
Cultures have been taken from the posterior tongue (anterior to the
foramen cecum), which is the most frequent area for candidal growth (1)
and from saliva (collecting 2 ml into test tubes). The devices containing
the samples have been incubated for 48 hours at 37 Celsius degrees and
positive candida carrier was based on one or more colony growth in
culture [4].
Statistical evaluations were used to analyze the findings: chi-square
test was used to evaluate the association between prevalence of candida
carriage and smoking. T-test was used to evaluate the association between
candidal carriage and amount and duration of smoking. Mann-Whitney
test was used to evaluate the association between candidal colonies count
in the study group versus the control. Spearman correlation was used to
evaluate the correlation between candidal colonies count and amount
and duration of smoking in the study group. p < 0.05 was required for
statistical significance.
Results
The associations between smoking and Candidal carriage are
presented in table 1. Although the prevalence of Candida positive cultures
was higher among smokers in saliva and/or tongue, (56% and 50%) no
statistical significance was found.
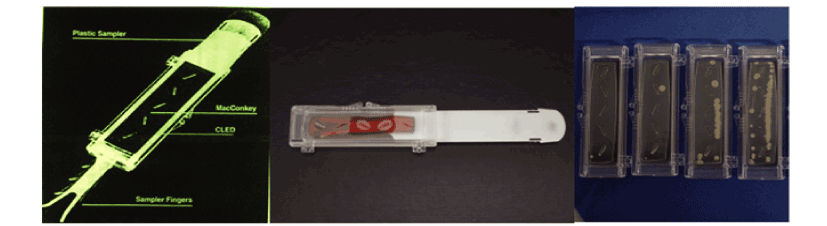
Figure 1: The original Diaslide device was a clinical tool for identification
of urine bacterial colonies, hence consisted the MacConkey agar as
medium.
The Diaslide device, modified by substitution of the growing media to
Agar Sabouraud, was used as a clinical tool to provide a quantitative
assessment of candida colonies. Instrument calibration was performed
based on known concentrations of Candida in order to enable the
comparison of different cultures.
The contribution of the duration of smoking and the daily amount on
the prevalence of Candidal carriage was examined. The findings failed
to show any significant trend in each one of those two parameters on
Candida carriage.
Candidal colonies count among smokers in association with the
amount and duration of smoking were examined, where 3 and 5 colonies
stand concentrations of over 103 (3 colonies) and closer to 104 (5 colonies)
respectively. No significant association was found between the number of
saliva\tongue colonies and smoking years or cigarettes consumption.
The associations between Candida colonies Count from study group
and control group is presented in table 2. The number of colonies in saliva
was the only significantly finding indicating higher prevalence of candida
in 50 smokers (mean 1.04 colonies) compared to only 0.5 in the 50 nonsmokers
(p=0.037). When those findings are transformed to CFU/mL, the
average colonies count from saliva in the study group was 100 CFU/mL
compared to less than 100 CFU/mL in non-smokers.
Discussion
There are several hypotheses brought in literature regarding possible
connection between smoking habits and oral Candida: mucosal
changes effecting the colonization of candida [9], pro-candidal
factors found in tobacco [13], the correlation between acidification of
saliva (caused in part by smoking) and carriage state of candida [20].
Despite these evidences, there is no uniformity in literature reports
regarding the correlation between smoking and oral Candida [3-11].
The present study had tested various parameters in order to describe
the association between tobacco smoking and oral candida. The effect
of duration and amount of smoking on the prevalence of candida was
examined. Comparison was also made between cultures from the back of
the tongue to those originating from saliva. We used the diaslide device
[18] modified to provide a quantitative assessment of candidal colonies.
The Sabouraud dextrose agar, proposed in 1894, is still the medium most
frequently employed in the primary isolation of pathogenic fungi [19]
and served as medium for the modified device in the present study. The
course of the current study resembles the course presented by Oliver and
his partners [4], since their study also included 100 participants (37 of
them were smokers), and demonstrated an association between candidal
colonies count and tobacco smoking, and no significant correlation
between candidal carriage and the habit of smoking [4].
| group |
n |
Carriage - saliva
samples (%) |
Carriage - tongue
samples (%) |
Carriage -
total (%) |
| study |
50 |
(54%)27 |
(42%)21 |
(56%)28 |
| control |
50 |
(44%)22 |
(28%)14 |
(50%)25 |
Table 1: Candidal carriage among smokers compared with nonsmokers,
i.e. associations between smoking and Candidal carriage.
| group |
Total number of colonies
originating from saliva |
Total number of colonies
originating from tongue |
| study |
52 |
26 |
| contro |
25 |
16 |
| P value |
0.037 |
0.132 |
Table 2: Candidal colonies count among smokers compared with
nonsmokers, i.e. associations between Candidal colonies Count from
research group and control group, read according to index (CFU/ mL).
102
/ml - One Colony
103
/ml - two colonies
104
/ml - Twenty colonies
105
/ml - above twenty colonies
The current study, as well as the study presented by Oliver and his
partners [4] might possibly indicate an association between Candida and
tobacco smoking developing while candidal carriage exists already prior
to the beginning of smoking, with tobacco only contributing to increased
concentration of yeast by inducing changes in mucous membranes,
releasing pro-candidal factors found in tobacco [13] and acidifying
salivary pH, as favored by candidal species [20].
This study suggests that smoking demonstrates a minor influence on
candidal colony growth, compared to other known factors such as dry
mouth, immunodeficiency disorders or antibiotic treatment.
The diaslide device was found very convenient to operate, yet possibly
limited as a quantitive measurement tool for candida. The device consists
of a hinged case containing two opposing agar media separated by a
sampler with a handle at one end and two bent sampler tips at the opposite
end. The tips of the sampler are first dipped into the sample. The sampler
is then pulled out through the casing, simultaneously inoculating both
agar surfaces. As a result, individual colonies can be observed even when
bacterial concentrations exceed 10 CFU/mL [6]. The number of colonies
on the diaslide correlates linearly with CFU per mL as determined
by dilution plating [18]. Its main disadvantage is that the results are
restricted to orders of magnitude. Furthermore, its narrow tip might be
limiting the anticipated result while taking a sample the tongue (in former
studies [4,9] higher counts were demonstrated from tongue). However,
further technical modifications might improve its efficacy. For example,
modifying the sampler tip to be broad and flat resembling tongue-scraper.
The average age of smokers in the study group was relatively low. A
more varied age group might demonstrate higher expression of the candida
carriers, but old age might express diverse factors such as compromised
health, prescribed medications, etc.
Another limitation of this study is a relative minority of participants
who have been smoking for decades and dozens of cigarettes a day
(most of the smokers in the study smoked less than ten years and
less than twenty cigarettes a day) and possibly a more varied group
of smokers, in terms of duration and amount of smoking, would
have a different impact on number of the count of candida colonies.
Another limitation of this study is that colonies of Candida were examined
without separating the different candida species. Duration and amount
of smoking may affect certain species more than others and investigating
this aspect of the association between Candida and tobacco smoking
might demonstrate valuable results.
Article Information
Article Type: Research Article
Citation: Becker T, Porat D, Meir Gorsky (2015)
The Association between Smoking Habits and
Candida in the Oral Cavity. Int J Dentist Oral Health
Volume1.2: http://dx.doi.org/10.16966/2378-7090.107
Copyright:© 2015 Becker T et al. This is an
open-access article distributed under the terms
of the Creative Commons Attribution License,
which permits unrestricted use, distribution, and
reproduction in any medium, provided the original
author and source are credited
Publication history:
Received date: 02 February, 2015
Accepted date: 25 March, 2015
Published date: 30 April, 2015


