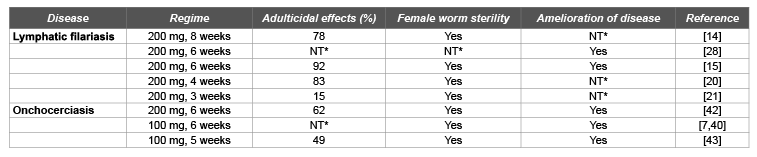
Table 1: Summary of trials using doxycycline to treat human onchocerciasis and lymphatic filariasis
NT*= Not tested
Kenneth Bentum Otabil1,2 Seth Boateng Tenkorang2 Ankrah Lennox-Mac2
1Department of Community Medicine and Health, Anglican University College of Technology, Nkoranza, BA, Ghana*Corresponding author: Kenneth Bentum Otabil, BSc. MPhil, Lecturer/Medical Research Scientist, School of Food and Health Sciences, Anglican University College of Technology, P.O Box 78 Nkoranza, BA, Ghana, West Africa, Tel: +233209164671; E-mail: i_chora@yahoo.com
Lymphatic filariasis and onchocerciasis are filarial diseases with potentially debilitating health outcomes as they collectively account for the loss of about 7.2 million disability adjusted life years in both men and women annually. Ivermectin, the major player in the mass drug administration (MDA) programme, has been effective at clearing microfilaria and halting transmission of these infections in some endemic communities. It is however unable to ameliorate the chronic conditions associated with the infections. When anti wolbachial therapy was discovered, an important milestone in the control of filariasis was chalked. Several investigators have since screened many anti wolbachial agents in varied combinations and regimes in order to find a drug/regime that could deplete Wolbachia, kill adult worms, deplete baby worms and ameliorate pathologies associated with these filarial diseases. This scoping review takes a critical look at the literature on anti wolbachial therapy and argues that doxycycline is by far the most promising of the current anti wolbachial drugs under investigation. This is in view of the fact that doxycycline depletes Wolbachia, kills adult worms and indirectly depletes baby worms. Doxycycline also ameliorates chronic pathologies such as lymphedema, hydrocele, dilation in scrotal lymphatics, thickening of the scrotal skin (associated with lymphatic filariasis) as well as dermatitis and ocular diseases (associated with onchocerciasis). Barring a few logistical challenges arising from contraindication in pregnant women and children below 8, doxycycline is relatively safe and does not generate adverse effects associated with rapid killing of worms as with some MDA drugs.
Doxycycline;Wolbachia; Endosymbiont; Filariasis; Onchocerciasis
Lymphatic filariasis and onchocerciasis are filarial diseases with potentially debilitating health outcomes as they collectively account for the loss of about 7.2 million disability adjusted life years in both men and women annually [1-4]. Ivermectin, the major player in the mass drug administration (MDA) programme, has been effective at clearing microfilaria and halting transmission of these infections in some endemic communities. It is however unable to ameliorate the chronic conditions associated with the infections.
The search for new antifilarial agents that could ameliorate filarial pathologies whilst halting transmission is ongoing. In more recent times, research efforts have focused on finding therapeutic agents that target Wolbachia endosymbionts of filarial worms [5-11]. This is because Wolbachia bacteria are essential symbionts of the major pathogenic filarial nematode parasites of humans including Wuchereria bancrofti, Brugiamalayi and Onchocerca volvulus [1] and are important both as chemotherapeutic targets and disease-causing organisms. Arguably, the most promising antiWolbachial agent is doxycycline, as its ability to clear infection and also ameliorate filarial pathology has consistently been proven by several investigators.
Doxycycline is a bacteriostatic tetracycline which can be given orally (the mode of administration adopted by several clinical trials in lymphatic filariasis and onchocerciasis) or parenterally for treatment. Doxycycline is almost completely absorbed from the gastrointestinal tract and about 93% is bound by plasma proteins. It has a half-life of 18 hours and is excreted by the kidneys making once-a-day dosing possible [12, 13].
Doxycycline is a bacteriostatic drug that inhibits protein synthesis in endosymbioticbacteria referred to as Wolbachia present in filarial nematodes including Wuchereriabancrofti, Brugiamalayi and Onchocerca volvulus[14]. Wolbachia spp. are abundant in all developmental stages of filarial nematodes and in the hypodermis and reproductive tissue of adult parasites. Embryogenesis and larval development in filarial worms are completely dependent on the presence of Wolbachia [1], thus, killing of Wolbachia leads to worm sterility and ultimately worm death.
According to Debrah et al. [15], doxycycline also reduces plasma VEGF-C and soluble VEGFR-3, thereby inhibiting angiogenesis in lymphoedema patients. More recent insights from the work of Fainaru and colleagues [16] further demonstrates that doxycycline prevents VEGF-induced vascular permeability resulting in less fluid accumulation in body organs such as the testes. Thus, in addition to its anti Wolbachial effects [14], doxycycline acts as a regulator of the factors responsible for proliferation of lymphatic endothelium and dilation of lymph vessels [16].
The present Mass Drug Administration (MDA) drug ivermectin has been effective in clearing baby worms (microfilariae) and has helped a lot in halting transmission of the diseases in some regions [17-19]. This microfilaricidal activity is especially beneficial in onchocerciasis because the microfilaria is responsible for onchocercal pathology. In the case of lymphatic filariasis however, ivermectin is able to kill the microfilaria but has little effect on the macrofilariae (adult worms) which are responsible for the pathology. There is thus the challenge of getting an additional drug which can deplete Wolbachia, clear baby worms, kill adult worms and provide amelioration for chronic sufferers of these debilitating diseases.
Several investigators set out to find just how far antiWolbachial therapy could be pushed [5-11]. After several clinical trials, it is evident that doxycycline could be the missing link in our effort to eliminate filariasis as it depletes Wolbachia, kills adult worms, depletes baby worms and improves the condition of individuals with chronic stages of both onchocerciasis and lymphatic filariasis.
Clinical manifestations of lymphatic filariasis are due largely to the mechanical obstruction of lymphatic vessels by the adult worms of W. bancrofti and the release of Wolbachia endosymbionts from dying adult worms. Thus, a drug that can effectively clear Wolbachia and consequently kill adult worms is critical, if pathology is to be halted or ameliorated.
The action of different regimes of doxycycline on the adult worms of W. bancrofti has been demonstrated by several investigators. Taylor and colleagues [14] demonstrated that an 8-week course of 200 mg doxycycline kills the adult worms by depleting Wolbachia. In their study, they observed a strong reduction (78%) in the number of worm nests in the scrotum and levels of filarial antigens in the blood [14].
Other studies using 6, 4 and 3 week doxycycline treatment followed by ivermectin and albendazole showed varied results. The macrofilaricidal activity of the 6 and 4-week regimens were comparable (92 and 83%, respectively) [15,20], whereas there was no macrofilaricidal activity in the 3-week group [21]. In the latter, the Wolbachia loads were only reduced by 80%, in contrast to the other regimens in which a more than 90% reduction of Wolbachia copy numbers were monitored by quantitative PCR. Thus, there seems to be a minimum cumulative dose of doxycycline required for a more than 90% Wolbachia reduction, which subsequently leads to a macrofilaricidal effect in lymphatic filariasis [8].
Although studies with Diethyl Carbamazine have indicated a partial activity against adult worms after 1 year [22-27], the unpleasant adverse effects that accompany its administration in areas co-endemic with onchocerciasis excludes its use in Africa and it has no ameliorative effects on disease pathology [26,27].
Lymphedema or elephantiasis is a chronic condition of lymphatic filariasis. Sufferers of this debilitating condition are often left with the option soft issue debridement or ‘accepting their fate’ as it were. However, it is not uncommon to find relapses even after a successful surgery. This is because current MDA drugs are unable to kill the adult worms which are long-lived; hence they continue to obstruct and destroy the lymphatic vessels and release more Wolbachia when they age and die.
The ability of doxycycline to alleviate lymphedema was thus investigated in search for relief for the sufferers. In a study by Debrah et al. [15], a 6-week course of doxycycline demonstrably affected the legs of all the doxycycline-treated patients and revert them to a lower lymphedema stage at 12 months post therapy, whereas all the placebo-treated patients stayed the same or changed to a higher stage at this time point. The amelioration in lymphedema manifested as better skin integrity (fewer “knobs”), reduction of deep and shallow skin folds, and fewer entry lesions of the affected legs [15].
Mand et al. [28] in a later study demonstrated that doxycycline treatment improved mild to moderate lymphedema independent of ongoing infection. The investigators opined that this finding expanded the benefits of doxycycline to the entire population of patients suffering from lymphedema. Thus, they concluded that patients with lymphedema stage 1–3 should benefit from a 6-week course of doxycycline every other year or yearly, and this should be considered as an improved tool to manage morbidity in filarial lymphedema or elephantiasis [28] (Table 1).
A common urogenital condition in filarial hydrocele is the dilation of lymphatic vessels of the scrotum. In a study by Taylor et al. [14] a 6-week course of doxycycline significantly reduced dilations in the lymphatic vessels 18-22 months post-treatment.
Otabil [29] using a 6-week course of doxycycline demonstrated that it improves dilations in the scrotal lymphatics and this improvement occurred even in patients with no active infection. The investigator was thus of the view that doxycycline has an additional mode of action apart from Wolbachia depletion as its effects were seen even in the absence of adult worms, the source of Wolbachia.
Earlier studies on the molecular mechanisms controlling the lymphatic vessels have established that the vascular endothelial growth factors (VEGF) C and D specifically control lymph angiogenesis in humans [30,31] by activating the VEGF receptor-3 (VEGFR-3) [32-35], which is principally restricted to the lymphatic endothelium in adult humans [36,37]. Jeltsch et al. [32], using animal models, demonstrated that the over-expression of VEGF-C in the skin of transgenic mice results in the proliferation of lymphatic endothelium and the dilation of lymph vessels similar to lymphatics infected with filarial parasites [38].
Doxycycline reduces plasma VEGF-C and soluble VEGFR-3 levels, thereby inhibiting angiogenesis in lymphedema patients. It also prevents VEGF-induced vascular permeability resulting in less fluid accumulation in body organs such as the testes [15,16].
Thus, in addition to its macrofilaricidal effects, doxycycline acts as a regulator of the factors responsible for proliferation of lymphatic endothelium and dilation of lymph vessels. The ability of doxycycline to reduce VEGF levels is an added advantage as it provides improvement even in patients without any active infection.
Filarial hydrocele is the accumulation of fluid in the scrotum following infection with lymphatic filariasis in some men. Current MDA drugs do not provide any improvement to those with hydrocele [26]. However, some studies have shown that doxycycline reduces the sizes of hydrocele in men with lymphatic filariasis.
In a study by Debrah et al. [15], a 6-week course of 200 mg doxycycline reduced the sizes of early-stage filarial hydrocele in patients with circulating filaria antigens (active infection). Interestingly, another study showed that even patients who had no detectable circulating filaria antigens, adult worm nests and baby worms, experienced significant improvement in their hydrocele sizes after a 6-week course of 200 mg doxycycline [29].
The ability of doxycycline to reduce hydrocele sizes could be attributed to its ability to reduce vascular hyper-permeability via down regulation of VEGF C and D [15], resulting in less fluid accumulation in the tunica vaginalis of the testes and the gradual killing of the adult worms [38] to prevent further damage.
In filarial hydrocele, it is consistently observed that skin of the scrotum thickens as the disease progresses [38]. Again, as the current MDA drugs do not provide improvement in the scrotal skin thickening, investigators tested the ability of doxycycline to provide improvement in the degree of scrotal skin thickening.
A study using a 6-week course of doxycycline demonstrated significant improvement in the thickening of the scrotal skin of doxycycline-treated patients (p=0.0120) when compared with the placebo-treated patients after 24 months [29]. The exact mechanism by which this improvement occurs is not known, although it may be due to the ability of doxycycline to reduce vascular hyper-permeability via down regulation of VEGF C and D. However, more research is needed in this regard [29].
The thickening of the skin of the scrotum has also been reported as an indicator of the risk to develop lymph scrotum [39]. Thus, when doxycycline ameliorates scrotal skin thickening, it also reduces the risk of developing complications associated with filarial hydrocele in the treated patients.
Hoerauf et al. [7,40] in some pilot, open-labeled trials in Ghana demonstrated that 6-week courses of 100 mg/day, oral doxycycline cause >90% reductions in Wolbachia levels from filarial tissues followed by an almost complete and sustained absence (12–18 months) of microfilaria from the skin. They observed deleterious effects of doxycycline on embryogenesis by histological assessment of extirpated nodules, although a clear adulticidal effect of doxycycline could not be determined in onchocerciasis patients after 18 months [7,40].
However, in some placebo, controlled, clinical trials, with extended follow-up analysis, it was proven that 4 or 6 week courses of 200 mg doxycycline [42] or a 5-week course of 100 mg doxycycline killed adult O. volvulus worms, 21–27 months after receiving treatment [43] (Table 1).
Clinical manifestations of onchocerciasis include both dermatologic and ocular disease [1]. The ability of doxycycline to kill adult worms of O. volvulus means that it is able to remove the source of Wolbachia and indirectly deplete baby worms.
Wolbachia appear to have a major role in the development of pathology in O. volvulus infections. Although manifestations such as blindness and dermatitis in onchocerciasis have been linked to the activity of the baby worms, some studies have demonstrated the role of Wolbachia in the development of these manifestations [1,40]. Since the baby worms induce both skin and eye disease in onchocerciasis, reduction in the baby worm load by doxycycline is correlated with amelioration of disease, except for already existing irreversible damages [8].
In this regard, it noteworthy that the current ‘wonder drug’ ivermectin has been highly effective in interrupting infection and clearing microfilaria in some endemic communities. Thus, it is able to reduce both skin and ocular disease. However, because it principally targets the baby worms, there is the need to sustain the treatment for extended periods to cover the reproductive lifespan of the long-lived adult worms and to be applicable to a large population in order to interrupt transmission.
Doxycycline is a relatively well-tolerated drug in the tetracycline class. In more than 1000 volunteers treated with doxycycline so far, there has not been any severe adverse effects [8] and there are a variety of strategies that can be used to reduce the incidence of some of the common sideeffects of doxycycline [12, 13].
However, doxycycline is contraindicated in those with allergy or sensitivity to the drug, pregnant and lactating women and children below 8 years because of its deleterious effects on bone and tooth development [12, 13]. This creates some logistical challenges in applying the drug to a wide population as the aforementioned groups will not be covered.
Again, the gold standard for doxycycline treatment in filariasis is a daily dose of 200 mg for 6 weeks. This relatively long duration of treatment creates further logistical challenges as the treatment must constantly be monitored for adverse effects. Such long antibiotic regimes are likely to increase the pace of the development of resistance.
Treatment targeting Wolbachial endosymbionts in filarial worms is becoming established as a treatment option in filariasis especially in cases where chronic pathologies have set in and current MDA drugs do not provide amelioration. Doxycycline by far is the most promising of the antiWolbachial drugs. This is because aside its ability to kill adult worms, it demonstrably ameliorates urogenital conditions of lymphatic filariasis such as scrotal skin thickening, dilations in scrotal lymphatic vessels, size of filarial hydrocele whilst halting or reversing progression of lymphedema and hydrocele. It also improves skin and ocular disease in onchocerciasis.
In spite of its long treatment duration (typically 6-weeks), doxycycline has limited side effects and has proven to be especially useful in areas where lymphatic filariasis or onchocerciasis is co-endemic with Loasis. This is because Loa loa, the causative agent of Loasis is free of Wolbachia symbiosis, and is unaffected by antiWolbachial treatments and thus avoids the risk of serious adverse events caused by rapid killing of baby worms [44-46].
Although logistical challenges may discourage the application of doxycycline as an MDA drug in the control of filariasis individual treatments with doxycycline has already started in some endemic communities. This is particularly so in areas where many chronic sufferers of these diseases live in search of a remedy to alleviate their suffering. Doxycycline can also be given to individual patients who have left a transmission area as it can achieve a strong reduction of the adult worm load in the absence of any re-infection.
In conclusion, research efforts should focus on how to fine-tune the current treatment regimes of doxycycline. In this regard, it is noteworthy that various combinations of doxycycline and other antibiotics/antifilarial agents have been and are still being investigated in human trials. In the long run, these trials will help unravel the full potentialities of the ‘secret weapon of the infectious disease Physician’ [13] that is doxycycline.

Table 1: Summary of trials using doxycycline to treat human onchocerciasis and lymphatic filariasis
NT*= Not tested
The authors have no conflicts of interest to declare.
KBO conceived the idea of the review, researched the literature and wrote the manuscript; SBT and LMA critically reviewed and revised the manuscript. All authors have read and approved the final manuscript.
KBO is a Lecturer/Research Scientist at the Department of Community Medicine and Health, Anglican University College of Technology, Nkoranza, BA, Ghana and a Research Scientist at Department of Medical Research, Emmavick Clinic, Sunyani, Ghana. SBT is a Research Scientist at Department of Medical Research, Emmavick Clinic, Sunyani, Ghana. LMA is a Scientist at Department of Medical Research, Emmavick Clinic, Sunyani, Ghana.
Download Provisional PDF Here
Article Type: Review Article
Citation: Otabil BK, Tenkorang SB, Lennox-Mac A (2015) The Case for Doxycycline in Our Battle against Filariasis. Autoimmun Infec Dis 1(2): doi http://dx.doi.org/10.16966/2470-1025.106
Copyright: © 2015 Otabil BK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access