Abstract
Background
Pemphigus vulgaris is an autoimmune blistering disease that involves the skin and mucous membranes. The antigen target is
desmoglein, a cadherin-type cell-to-cell adhesion molecule found in desmosomes. The levels of immunoglobulin G anti-desmoglein 3 antibodies
correlate positively with disease activity. Furthermore, the immunoglobulin G4 isotype appears to play a predominant role in pathogenesis.
However, anti-desmoglein 3 antibodies are present in patients with pemphigus vulgaris during clinical remission. Our objective was to measure
and compare the immunoglobulin G4 response to desmoglein 3 in patients with pemphigus vulgaris in clinical remission with those with clinically
active disease.
Methods
We included sera from 7 male and 10 female caucasian patients with an age range of 35-76 years. Patients were previously diagnosed
with pemphigus vulgaris (12 with clinically active disease, 14 in clinical remission) and who were positive for anti-desmoglein 3 autoantibodies
by enzyme-linked immunosorbent assay were studied. Anti-desmoglein 3 (total immunoglobulin G, immunoglobulin G1, and immunoglobulin
G4 subclasses) antibodies were measured by modified enzyme-linked immunosorbent assay. Also, immunoglobulin G subclasses of anti-skin
antibodies were tested by indirect immunofluorescence.
Results
The anti-desmoglein 3 specific immunoglobulin G1 and immunoglobulin G4 subclass study revealed that sera from patients with
clinically inactive disease have lower anti-desmoglein 3 immunoglobulin G4 subclass antibody levels than sera from those with active disease by
enzyme-linked immunosorbent assay (p=0.03). However, there was no statistically significant difference for immunoglobulin G1 between the two
groups. Similarly, the presence of immunoglobulin G subclasses of anti-skin antibodies by indirect immunofluorescence between the two groups
was not statistically significantly different.
Conclusion
Levels of anti-desmoglein 3 immunoglobulin G4 subclass autoantibodies look very adequate to compare patients in remission
with clinically active patients with pemphigus vulgaris (p=0.03).
Keywords
Pemphigus vulgaris; Desmoglein; Immunoglobulin G; Anti-skin antibodies; Enzyme-linked immunosorbent assay; Indirect
immunofluorescence; Clinical remission; Autoantibodies
Introduction
Pemphigus vulgaris (PV) is an autoimmune disease that causes
blistering of the skin and mucous membranes. The autoimmune target of
pemphigus is desmoglein, a cadherin-type cell-to-cell adhesion molecule
found in desmosomes [1,2]. The desmoglein 1 (Dsg1) and desmoglein
3 (Dsg3) isotypes are found in the stratified squamous epithelia where
blisters form [1]. The development of an enzyme-linked immunosorbent
assay (ELISA) for Dsg1 and Dsg3 has led to advancements in the
classification of pemphigus [3,4]. Patients with PV have anti-Dsg3 and
anti-Dsg1 IgG antibodies, and some patients have only anti-Dsg3 IgG
antibodies [5-7].
In addition to the identification of pemphigus subtypes based on the
antibody profile, the quantification of desmoglein antibodies correlates
positively with the clinical activity of the disease. In patients with PV,
quantitation of Dsg3 antibodies by ELISA parallels the clinical activity
of the disease [6,8]. Several studies have shown that the participation
of the IgG subclasses in the pathogenesis of PV is very different, with
IgG4 having a predominant role. Different IgG subclasses associate with
different functional properties and may thus determine the pathogenic
potential of IgG antibodies [3,9-11]. In active PV, IgG4 autoantibodies
against Dsg3 were found to predominate. An IgG4 response is necessary in
order for clinical disease to develop. On this basis, complement activation
is not required for blister formation. Moreover, the total amount of serum
IgG4 has been shown to increase in pemphigus [12].
These data suggest that IgG4-targeted therapies for pemphigus could
have a beneficial effect. Intravenous immunoglobulin therapy lowers the
levels of IgG4 anti-Dsg3 antibodies, and this effect correlates with improved
clinical activity [13]. Additionally, the good results obtained in pemphigus
and other diseases treated with B lymphocyte-depleting therapies are
related to decreased levels of serum IgG4 or Dsg3 autoantibodies [14,15].
Despite that the levels of IgG4 anti-Dsg3 autoantibodies correlate
with the clinical activity of PV in patients, the presence of anti-Dsg3
autoantibodies has been found in patients in clinical remission [6,11].
These data call the usefulness of these assays in assessing disease activity
into question. Also, the meaning of these autoantibodies during clinical
remission is a matter of discussion [16].
The aim for the study was to assess the IgG subclass composition of
anti-Dsg3 antibodies in two groups of patients, clinical remission patients
compared with patients who have clinically active disease. We evaluated
levels of IgG1 and IgG4 anti-Dsg3 antibodies in both groups by ELISA.
Sera from patients with PV in clinical remission contained lower IgG4 antiDsg3
antibody levels than sera from patients with clinically active disease
(p=0.03). We also compare these data with the IgG subclass composition
of anti-skin antibodies (ASA) by indirect immunofluorescence (IIF).
Materials and Methods
Human Sera
We collected sera from 7 male and 10 female caucasian patients with
an age range of 35-76 years previously diagnosed with PV by clinical
presentation, histology, and positive serological test. We selected 26 serum
samples positive for anti-Dsg3 autoantibodies by ELISA. The project was
done under the approval of Ethics in Research Committee of Universidad
de Navarra (nº 79/2010).
Sera were classified into two groups depending on the activity of each
patient’s disease (supplementary information). The first group consisted
of 12 serum samples taken at a situation of clinical activity, characterized
as the presence of several active lesions on the skin and/or mucous
membranes prior to any treatment. The second group included 14 serum
samples collected in clinical remission, which was defined as an absence
of active lesions on the skin and/or mucous membranes while the patient
received treatment with low-dose (less than 15 mg per day) corticosteroids
as maintenance treatment.
The treatment given to patients in the second group was as follows: highdose
(more than 15 mg per day) corticosteroids (patients P1, P6, P8, P9,
P13 and P14), rituximab, 4 weekly intravenous doses of 375 mg/m2 each
(patients P4, P5, P12, P15 and P16), or intravenous cyclophosphamide
pulse therapy (patients P2, 7 doses of 1000 mg per dose; P7, 3 doses of
1700 mg per dose; and P10, 10 doses of 1200 mg per dose).
To establish the cutoff values for anti-Dsg3 IgG1 and IgG4 subclasses
by ELISA, 20 sera from healthy persons were selected as controls. All sera
were stored at −20°C.
Detection of Dsg3 antibodies by enzyme-linked immunosorbent
assay
Total IgG autoantibodies against Dsg3 were quantified using a
commercial Dsg3 ELISA Kit (Euroimmun, Luebeck, Germany) according
to the manufacturer´s instructions. Anti-Dsg3 IgG1 and IgG4 subclasses
were quantified using the same commercial Dsg3 ELISA Kit (Euroimmun,
Luebeck, Germany), except that the mouse antihuman IgG1 or IgG4
conjugated with horseradish peroxidase (Beckman Coulter Inc, Fullerton,
California) was used as the secondary antibody
Serum samples were serially diluted starting 1:100. The lowest dilution
measurable in the optical density (OD) range at a wavelength of 450 nm
was determined as the optimal serum dilution. The value for each serum
was calculated by multiplying the OD value at the optimal serum dilution
by the dilution factor. The IgG1 conjugate was diluted 1:1000 and the IgG4
conjugate was diluted 1:3000. All dilutions were made with sample buffer
provided in the commercial Dsg3 ELISA Kit (Euroimmun, Luebeck,
Germany).
Assay values were considered positive when the IgG ELISA was greater
than or equal to 20 relative units (RU)/mL, the cutoff recommended by
the manufacturer (Euroimmun, Luebeck, Germany). The cutoff values for
anti-Dsg3 IgG subclasses were 0.643 OD for IgG1 and 0.237 for IgG4 at
a wavelength of 450 nm. These cut off points were established based on
analysis of values in 20 control serum samples. The values of the cutoffs
were equal to the mean plus 2 standard deviations (SD) of the control values.
Indirect immunofluorescence
Anti-skin antibodies were detected by standard IIF on monkey
esophagus substrate (A. Menarini Diagnostics, Florence, Italy). The
staining procedure employed was as follows: serial serum dilutions (from
1/10 to 1/640) were applied to sections for 30 minutes (min). The slides
were washed in phosphate-buffered saline (PBS) for 10 min using a
magnetic stirrer and were incubated for 30 min with a secondary antibody.
A final wash of 10 min was performed and mounting medium included.
Primate anti-human IgG FITC-conjugate (Inova Diagnostics Inc, San
Diego, California) was used as the secondary antibody for ASA analysis.
For IgG subclass staining, mouse anti-human IgG1 or mouse anti-human
IgG4 FITC-conjugated monoclonal antibodies (Beckman Coulter Inc,
Fullerton, California) were used as secondary antibodies.
Statistical analysis
Statistical analysis was performed with IBM SPSS Statistics v20
software. Normality of the dataset was analyzed with the Shapiro-Wilk
test. Based on these results, we performed a non parametric (MannWhitney
U) test to compare variables. The linear relationship was studied
with the Spearman’s rank correlation coefficient. In all cases, a p-value
<0.05 was considered significant.
Results
Quantification of anti-Dsg3 antibodies by ELISA
Sera from patients with clinically active PV had higher IgG anti-Dsg3
values (median=254.5 RU/mL) than serum samples from patients with
clinically inactive disease (median=178.0 RU/mL) (p=0.04 by U MannWhitney
test, Table 1). Also the IgG4 anti-Dsg3 antibody levels were
different between the two groups. Patients with clinically active disease
had higher values (median=2.28 OD) compared with patients in clinical
remission (median=0.68 OD) (p=0.03, by U Mann-Whitney test, Table
1). However, there was no statistically significant difference in IgG1 levels
between the two groups (median=0.75 OD and 0.71 OD, respectively).
To summarize, serum samples from patients with clinically inactive PV
had lower values of total IgG and IgG4 subclass anti-Dsg3 than sera from
patients with clinically active disease (Figure 1).
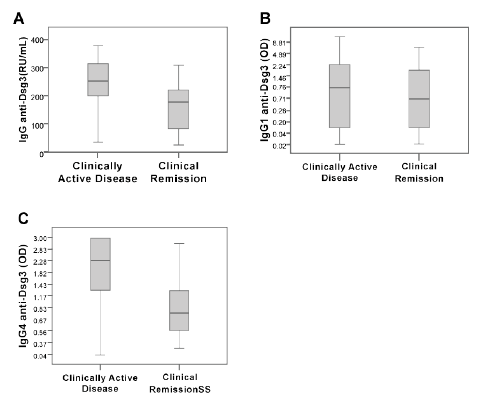
Figure 1: ELISA determination of anti-desmoglein 3 antibodies (antiDsg3)
in sera from patients with clinically active pemphigus vulgaris and
patients in clinical remission. We measured total IgG (A) and subclasses
IgG1 (B) and IgG4 (C). Data are showed by a Box Plot. The horizontal
line represents the median of relative units (RU)/mL or optical densities
(OD) measured at 450 nm.
Detection of IgG total and subclasses of ASA antibodies by IIF
We analyzed data from IIF to detect IgG, IgG1, and IgG4 ASA. The
serum dilution with a typical pattern of fluorescence of pemphigus vulgaris
(staining of intercellular spaces in monkey esophagus) was determined as
positive. We compared data from patients in clinical remission with those
experiencing a period of clinically active disease (Figure 2). There were
no statistically significant differences between clinical remission patients
and clinically active patients. Only ASA-specific IgG4 trended towards
significance (p=0.05 by U Mann-Whitney test, Table 1).
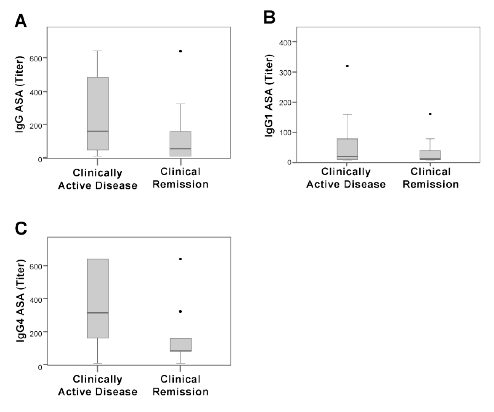
Figure 2: IIF determination of anti-skin antibodies (ASA) in sera from
patients with clinically active pemphigus vulgaris and patients in clinical
remission. We measured total IgG (A) and subclasses IgG1 (B) and
IgG4 (C). Data are showed by a Box Plot. The horizontal line represents
the median of titer.
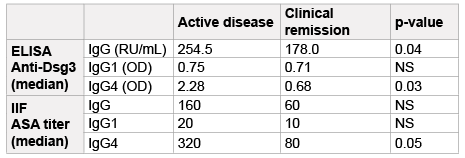
Abbreviations: ASA: Anti-skin Antibodies; Dsg3: Desmoglein 3; ELISA:
Enzyme-linked Immunosorbent Assay; IIF: Indirect Immunofluorescence;
NS: No Significant; OD: Optical Density; RU/mL: Relative Units per Milliliter
Table 1: Anti-Desmoglein 3 and Anti-Skin Antibodies in Patients with
Pemphigus Vulgaris
Correlation between modified ELISA with conventional IIF
Data from the IIF and ELISA showed a very good correlation. The
values of both IgG1 and IgG4 anti-Dsg3 and ASA IgG1 and IgG4
antibodies showed a positive correlation (R=0.74 and 0.75 for IgG1 and
IgG4 values, respectively).
Discussion
We compared the levels of IgG1 and IgG4 anti-Dsg 3 antibodies by
ELISA in sera from patients with PV in clinical remission with levels
from patients with active disease. We also studied the distribution of IgG
subclasses in ASA in the two groups of patients by IIF. The levels of antiDsg3
IgG4 subclass antibodies detected by ELISA were lower in patients
in clinical remission, differentiating patients with clinically active PV
from those with inactive disease. The IgG subclasses of ASA detected by
IIF did not reach statistically significant differences between the groups.
The clinical evaluation of patients has been easier since the identification
of Dsg3 antibodies detected by ELISA because these data correlate
with the clinical activity of the disease [8,17,18]. Several therapeutic
approaches are used to treat PV, including intravenous immunoglobulins
or B lymphocyte depletion, which have been successful because of their
effects on the level of anti-Dsg3 autoantibodies [13-15]. Moreover, the
distinction between IgG subclasses provides a better understanding of the
pathogenesis of this disease [3,9]. IgG4 has been implicated as the IgG
subclass responsible for the tissue damage in PV [19].
However, it has been repeatedly reported that numerous pemphigus
patients present with anti-Dsg 3 antibodies during clinical remission
[6,11,16,20,21]. These observations raise interesting questions about
the value of Dsg3 antibody measurements in the evaluation of clinical
activity. The role of these autoantibodies during periods of clinical
remission is unknown, although several possibilities are proposed. One
is that the autoantibodies remaining in circulation during remission are
predominantly non-pathogenic rather than pathogenic [16,22]. Another
explanation is that the immunosuppressive treatments used lead to a
symptomatic suppression of the disease while also being insufficient to
completely block the autoantibody response [16].
The presence of anti-Dsg3 antibodies in patients in clinical remission
together with the described pathogenic role of anti-Dsg3 IgG4 subclass
antibodies led us to test whether the levels of anti-Dsg3 IgG4 subclass
antibodies differ in patients with PV in clinical remission compared with
those of patients whose disease is clinically active. We show that the levels
of anti-Dsg 3 antibodies are lower during clinical remission with respect
to levels during active disease as previously reported [6]. In this report,
the authors claimed that monitoring the levels of these autoantibodies in
patients with PV in clinical remission on a regular basis could be useful
for assessing disease activity. Our study adds information, showing that
the levels of IgG4 anti-Dsg3 antibodies are also lower in patients with
PV during clinical remission. In a group of heterogeneous PV patients
in clinical remission which include anti-Dsg3 negative patients, Dhandha
et al. showed that there is not a subclass switch, and that these patients
maintain high levels of both IgG4 and IgG1. We found that the levels of
IgG4 anti-Dsg3 are lower in patients in clinical remission, but that there
was no change in the level of IgG1 in patients in remission with respect
to those with clinically active disease. Perhaps these differences occurred
because our patients are more homogeneous concerning the positivity of
Dsg3 antibodies during clinical remission.
These findings have particular relevance because of the well known
pathogenic role attributed to IgG4 antibodies in different pathological
conditions [3]. The decisive role of IgG4 antibodies in blister formation
has been documented in patients with PV [9,10]. On the other hand,
the beneficial effects of new therapeutic measures on the clinical disease
activity of PV correlate with the levels of IgG4 autoantibodies. Intravenous
immunoglobulin treatment ameliorates PV symptoms, inducing a
decrease in anti-Dsg3 IgG4 subclass antibodies [13]. Moreover, B
lymphocyte-depleting therapy such as anti-CD20 monoclonal antibodies
produces a clinical benefit in patients with PV that is associated with a
decrease in anti-Dsg3 antibodies [15].
Interestingly, the effect of anti-CD20 therapy in IgG4-related pathology
is linked to decreases in serum IgG4 levels [14]. Our data showing a
decrease in IgG4 anti-Dsg3 autoantibodies in patients with low clinical
activity could help to better understand these findings. No statistically
significant differences in the IIF data were observed between patients with
PV according to the presence or absence of clinical disease activity. Only
the titer of ASA-IgG4 subclasses trended towards a significant difference
(p=0.05). This could be due to the lower sensitivity with respect to ELISA,
and the different characteristics of both assays.
Measurements of IgG4 anti-Dsg3 autoantibodies appear to be a good
marker when antibody levels are to be correlated with the degree of
clinical activity of PV. Furthermore, the use of biological parameters, such
as the detection of IgG 4 anti-Dsg 3, look very adequate for interpreting
the efficacy of a given new treatment in patients with PV. Additionally, the
pathogenic significance of the presence of these antibodies when there is
no clinical activity deserves further research.