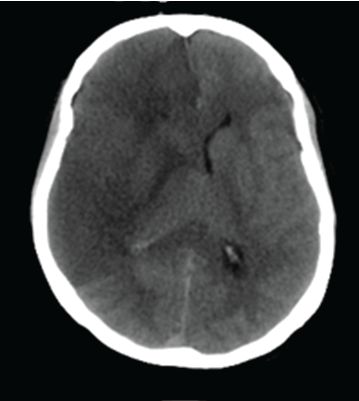
Figure 1: Computerized Tomography of cytotoxic brain edema in the left hemisphere following stroke.


Hans von Holst1,2* Pasi Purhonen3 Daniel Lanner2 Ramakrishnan Balakrishnan Kumar3 Hans Hebert3
1Section of Neurosurgery, Karolinska University Hospital, Sweden*Corresponding author: Hans von Holst, Department of Biosciences and Nutrition, Karolinska Institute and School of Engineering Sciences in Chemistry, Biotechnology and Health, KTH Royal Institute of Technology, Huddinge, Sweden, E-mail: hvh@cenesy.com
Increased intracellular water content defined as cytotoxic brain tissue edema is a serious secondary clinical complication to traumatic brain injury (TBI) and stroke and without knowledge to the etiology. Recently a hypothesis to the nervous tissue edema was presented suggesting that external dynamic and internal mechanical static impact forces caused protein unfolding resulting in an increased brain tissue water content. The hypothesis was confirmed by computer simulation tests. In this laboratory study we further evaluated the hypothesis by using the mature protein laminin LN521 upon the effects of both dynamic as well as static impact forces, respectively. Laminin was chosen as a representative protein due to it´s general and abundance presence in the cells. The treated laminin solutions were then analyzed with denatured electrophoresis and Electron Microscopy showing aggregation and fragmentation of the laminin structures. The present results confirm earlier hypothesis and computer simulation suggesting for the first time that dynamic impact force in an accident and increased mechanical static force in stroke unfold mature proteins having the potential to increase the intracellular water content defined as cytotoxic brain tissue edema. The clinical condition resembles the phenomenon when elasmobranchs including white sharks prevent their cells from too high hydrostatic pressure in the deep sea. Thus, the present laboratory study results and knowledge from marine physics may be considered to improve the clinical treatment and outcome of TBI and stroke patients.
Traumatic brain injury; Stroke; Cytotoxic brain tissue edema; Proteins; Adult brain injury
With a worldwide population showing increasing interest in various leisure activities including hockey, football and boxing, to mention just some, the number of injuries including trauma and stroke will be substantial in the next decade. One of the most severe secondary clinical complications in traumatic brain injury (TBI) and stroke is cytotoxic brain tissue edema (Figure 1) defined as intracellular swelling of the neurons, glial and endothelial cells found in both grey and white matter [1]. The concept of cytotoxic brain tissue edema seems to be more complex than expected, although the pathophysiological mechanisms to some extent have been proposed [2]. It is generally looked upon as a dysfunction in cellular metabolism between the extra and intracellular compartments mainly as a result of increased intracellular water content [3]. With increased intracellular water content the brain tissue is facing an unacceptable high intracranial pressure resulting in reduced regional cerebral blood flow, reduced oxygen supply and increased strain level in the nervous tissue thereby jeopardizing the cerebral metabolism further. The problem of cytotoxic brain tissue edema is especially reflected by the suboptimal neurosurgical decompressive craniotomy treatment in the most severe cases aiming at reducing the intracranial pressure to alleviate the secondary complications resulting in such a poor prognosis. A new hypothesis to the etiology to cytotoxic brain tissue edema has recently been proposed [4]. The hypothesis stipulates that following dynamic impact force to the head and internal mechanical static force following stroke results in the disruption of non-covalent and covalent bonds in proteins verified by computer simulation studies [4-6]. The unfolded proteins attract water molecules resulting in cytotoxic brain tissue edema with both increased strain level and intracranial pressure. Also, the disruption of nucleotides such as adenosine-triphosphates causes a dysfunction in ion hemostasis. Thus, both the increased intracellular water content and nucleotide disruption may tentatively explain the etiology to the cytotoxic brain tissue edema. All proteins influenced by the impact may participate in the edema formation. Thus, a generally existing protein such as laminin was chosen for the present study. The laminins are glycoproteins of 400- 900 kDa and found as a major component of basal lamina in most cells of the human body including brain tissue. The main function of laminin is gluing the cells to each other [7]. Lack of laminin will change the anatomy of cells causing further dysfunction of the cellular metabolism. In marine physics it has been shown that elasmobranchs including white sharks unfold protein structures to compensate for the increased hydrostatic pressure coming from outside when diving into the deep sea and thus interfering with the intracellular compartments [8]. The unfolding of protein structures increases the intracellular water amount by the hydrostatic pressure. By studying the cytotoxic brain tissue edema following TBI and stroke, better insight toward the etiology and, hence, its treatment should tentatively be supported by studying the protein metabolism in white sharks and other elasmobranchs in the ocean. If the earlier hypothesis and computer simulation is true, disruption, aggregation and fragmentation of the protein laminin LG521 should be obvious with the present laboratory investigation. The aims of the two laboratory experiments following both dynamic impact force and mechanical static pressure force were to analyze the presence of protein unfolding in a total amount of sixteen samples of laminin LN521 evaluated with denaturing electrophoresis and electron microscopy (EM), respectively.

Figure 1: Computerized Tomography of cytotoxic brain edema in the left hemisphere following stroke.
The basal metal plate including a centrally located 0.2 ml volume space was filled with an equal amount of a Laminin LN521 solution (BioLamina AB). Another metal plate was anchored on top of the basal metal plate. The double metal set was then stabilized on a bottom plate (Figure 2a) and impacted by a Hybrid III head form using a helmet drop test rig (Figure 2b) [9]. The drop height of the dummy head was set to 57 cm resulting in an impact speed of 3.1m/sec or 11.2 km/h. The dummy head form was guided through the drop and released before impact to get a controlled contact point toward the centrally located metal set including the laminin solution. The dummy head is equipped with a system of nine accelerometers [10] mounted inside the dummy head form. Three of the accelerometers were used to measure the translational accelerations of all axes. The acceleration data was then filtered using a 25000 Hz CFC 1000 filter. In all, eight dummy head impacts were performed to the eight laminin solutions inside the metal set. As controls, two laminin solutions were placed in the metal set, however without the dynamic impact force tests before analyzed. After each impact the metal set was opened to collect the laminin solutions for further analysis with denatured electrophoresis and EM, respectively. In the meantime, the impact results from the test rig were analyzed.
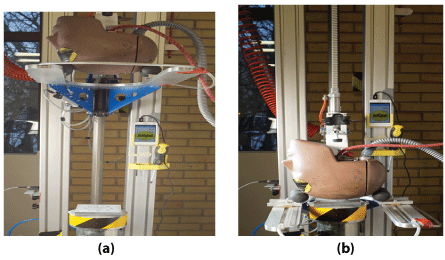
Figure 2: The dummy head before (a) and after (b) the impact towards the white metal set including the centrally located laminin solution.
In the second experiment the same metal plate was used and filled with a 0.2 ml solution of the protein laminin LG521 before an integrated pressure sensor analyzed the volt output and transferred to mmHg [11]. Zero Volt=zero mmHg as controls, 1 Volt=15 mmHg, 2 volts=30 mmHg, 3 Volts=45 mmHg and 4 Volts=65 mmHg for a period of 15 minutes, respectively. Each of the volt experiments included two solutions and thus two controls and eight solutions with the static pressure force. After exposure to each voltage, all ten samples were collected and further analyzed with denaturing electrophoresis and EM.
The samples were analyzed by denaturing polyacrylamide gel electrophoresis (PAGE). In denaturing electrophoresis, both the treated and untreated laminin samples were mixed with 5x loading buffer containing sodium dodecyl sulphate (SDS) and heated at 65°C for 10 minutes before loading on the 4-20% Tris-glycine gel (Thermo Fisher Scientific) [12]. The Electrophoresis was then started by applying 150 V and ran until the blue front reaches the end of the gel [13]. The bands in the gel were visualized by silver staining using Silver quest silverTM staining kit (Thermo Fisher Scientific).
1-3 µl aliquots of the impacted specimen were applied to glowdischarged carbon-coated copper grids, blotted and stained 30 s with 2 % (w/v) uranyl acetate [14,15]. The grids were checked using JEOL JEM-2100f transmission electron microscope (JEOL, Japan) operated at 200 kV. Images were collected with TVIPS TemCam-F415 4k × 4k CCD-camera (Tietz Video and Image Processing Systems GmbH, Gauting, Germany) using a nominal magnification of 80,000.
Translational acceleration: The resultant translational accelerations of the eight impacts to the laminin samples ranged between 473,7 and 535,5 g with a mean acceleration of 519,8 g within 0.004 seconds from dynamic impact. As expected, the oscillation following the peaks for all dynamic impacts is a result of vibrations in the accelerometer mount (Figure 3).
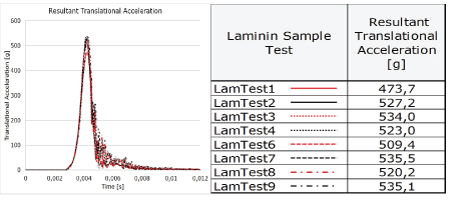
Figure 3: The translational accelerations in eight dummy head impacts (left) to the laminin solutions and results of the eight laminin sample test accelerations (right).
Electrophoresis: Denaturing PAGE was used to analyze the effect of force treatment on laminin. SDS, an amphipathic detergent, was used to denature the laminin. The hydrocarbon tail of SDS binds with laminin and exposes the buried hydrophobic region. The folded tertiary structure of laminin would be broken by the sulfate end of SDS. After mixing the sample with loading buffer containing SDS and heating it, the laminin was fragmented to subunits. The PAGE acts as a molecular sieve with smaller subunits migrating faster than the larger subunits. The eight force treated samples of which four laminin results are shown in Lanes 2-5 were considerably different than the control sample without force treatment as shown in Lane 1 (Figure 4). Fragmentation of laminin was observed in all the dynamic force treated samples (Lanes 2-5). A majority of the fragmented subunits of dynamic force treated laminin (Lane 2-5) were around 45 kDa (indicated as *, Figure 4) which could be laminin subunit beta 2 fragments containing Domain alpha (around 3 kDa) and 38.5 kDa Domain I. Another major band was around 66 kDa (indicated as ¤, Figure 4) and which could be domain II & I from laminin subunit gamma 1 that has a calculated molecular weight of 65 kDa.
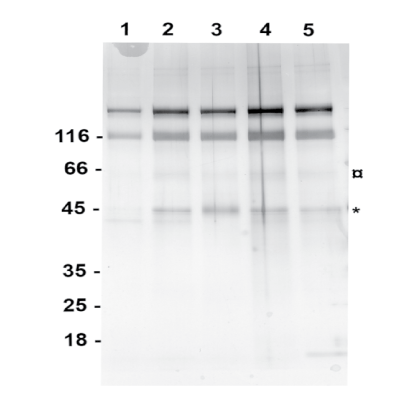
Figure 4: Denaturing PAGE analysis showing the fragmentation of laminin due to dynamic impact force. Lane 1 is the control sample of laminin without impact fore and Lane 2 to 5 are the laminin samples after impact force treatment.
The fragmentation is not seen in Lane 1 containing the control sample. This indicates that fragmentation of laminin was induced by the force treatment. Comparing the band pattern between samples with and without dynamic force treatment in denaturing gel (Figure 4) demonstrates the effect of force treatment on fragmenting the protein that could change the arrangement of water molecule around the laminin leading to aggregation.
Electron microscopy (EM): Laminin was easily visualized by negative stain electron microscopy due to its large molecular size. The untreated control proteins showed as long threads with attached globular regions (Figure 5a) depicting the arms and globular domains found in the laminin structure. Occasional self-assembly of laminin into single, approximate hexagonal structures was observed in both control and force-treated specimens. As compared to the control, the force-treatment increased protein aggregation (Figure 5b). At the same time part of the laminin was still found in linear arrays.
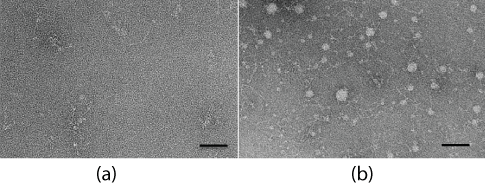
Figure 5: Electron micrographs of a negatively stained control (a) and a force-treated specimen (b), showing strong protein aggregation. Scale bars are 50 nm.
Electrophoresis: Denaturing PAGE was used to analyze the effect of static pressure force on the eight laminin samples of which half the number is presented in (Figure 6). When comparing the fragmentation pattern of the control sample in Lane 5 with the static pressure force samples in Lane 1 to 4 and treated with 1 to 4 volts, respectively, for 15 minutes, it is evident that increased static pressure force treatment has created new fragments and with the most prominent band seen in Lane 3 and 4 and denoted as ¤ (Figure 6). However, the new fragments were less pronounced compared to the results following dynamic impacts. As mentioned in section B, disulfide bonds can be broken only by reducing agents in vitro in denaturing SDS PAGE and running the gel without reducing agent would preserve the disulfide bonds. On comparing the result from previous denaturing PAGE on dynamic impact force tests with the static pressure force tests, one can safely conclude that the new fragments seen in the test samples are formed due to broken disulfide bonds in laminin as a consequence to the applied static pressure force. Such fragmentation in SDS PAGE due to disulfide bonds are always associated with protein aggregation [16] and which was clearly seen in Section B&C following dynamic impact force.
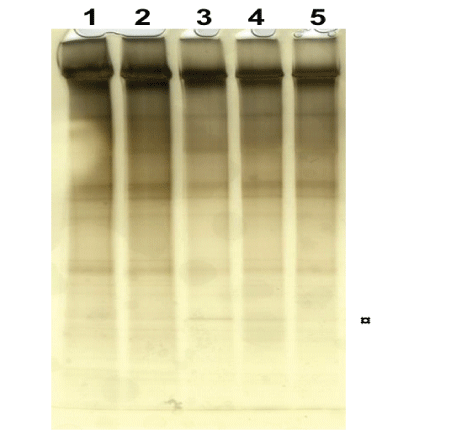
Figure 6: Denaturing PAGE analysis showing the fragmentation of laminin due to static pressure force in Lane 1-4 after treatment with 1 to 4 volts, respectively, for 15 minutes and compared with the control sample in Lane 5.
Electron Microscopy (EM): The use of mechanically static pressure force also generally increased the aggregation of laminin (Figure 7) as evaluated with EM. At the lowest static pressure force tested, 1 volt translated to 15 mmHg, no major difference was observed as compared to the untreated control specimen (Figure 7a), whereas the use of 2 volts and translated to 30 mmHg pressure force produced a slight increase in protein aggregation (Figure 7b). The most obvious effect was detected with higher static pressure impact forces of 3 volts translated to 45 mmHg and 4 volts translated to 65 mmHg, and where numerous protein aggregations could be seen (Figure 7c).
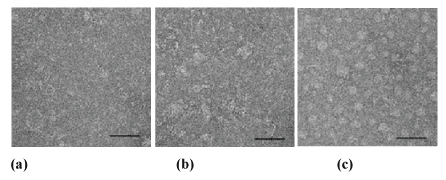
Figure 7: The effect of static pressure force on laminin as illustrated by negative staining EM. a) The control specimen without static impact force and specimen exposed to static impact force of b) 2 volts and c) 4 volts. Scale bars 50 nm.
Laminin was chosen because of its widespread precence in the human body should be looked upon as representative for protein structures when it comes to evaluate molecular bonding following impacts. Our results show for the first time that following a dynamic impact force of 3.1 m/sec or 11.2 km/hour and a mechanical static pressure force of 30 mmHg and higher, protein structures are unfolded and presented as aggregation and fragmentation evaluated by denatured electrophoresis and EM. The results should have significant influence when explaining the consequences of TBI and stroke at the clinical treatment of increased strain and intracranial pressure due to cytotoxic brain tissue edema. In this regard the metabolism from white sharks should be considered when it comes to develop new drugs in clinical neurosurgery. If the protein metabolism is negatively influenced by dynamic impact forces in such a low speed and by low mechanical static pressure forces as shown here, respectively, it seems natural to suggest that the metabolism should be even more jeopardized with even higher dynamic and static forces. Recently it was shown that postmortem analysis of football players presented evidence of chronic traumatic encephalopathy [17]. Also, in clinical neuroscience an increased intracranial pressure of around 30 mmHg and above following TBI and stroke may result in an unacceptable swollen brain. The ultimate choice of neurosurgical treatment is then decompressive craniotomy aiming at reducing the intracranial pressure. Although the number of deaths has reduced with this neurosurgical treatment, the number of disabled patients has on the other hand increased [18]. Thus, both dynamic and static forces to the head and the nervous tissue demand better understanding of the consequences aiming at improvement of the clinical treatment.
The stability of protein structures is influenced by attractive and repulsive forces including the balance of non-covalent and covalent bonding in switching between folded, intermediate and unfolded states [19,20]. Disturbance of its normal environment due to dynamic and static forces may cause instability and thus favors unfolding of the proteins. An impact to the head may result in switching from folded toward unfolded protein structures with an increased accumulation of water molecules to cover parts of the unfolded and hydrophobic structures [21]. The water in cells can be looked upon as one of the major parts being responsible for a number of activities in the cellular metabolism [22]. The structure and hydrogen bonding of intracellular water is essential for especially protein metabolism as it may have an extensive input on the intracellular volume following protein changes [23]. However, unregulated water accumulation in the nervous tissue may become devastating.
It seems natural to believe that protein structures including laminin at the impact location and its vicinity are disrupted causing disintegration between cells loosing not only their tight connections to each other but also the cellular metabolism. Also, the dynamic impact force at the accident together with the following increased strain level and mechanical static pressure force, the unfolded protein structures will attract water molecules in and around the disrupted proteins. The consequence is an increased water accumulation of the intracellular domains potentially resulting in what is defined as cytotoxic brain tissue edema. When high enough the increased strain level and intracranial pressure in the nervous tissue is defined with neuroimaging analysis together with clinical deterioration of the patient. In the present results it was clearly shown that the translational acceleration around 500 g during some milliseconds had the capacity to unfold the laminin structure into aggregated and fragmented structures even at the low impact of 3.1 m/sec or 11,2 km/hour. This dynamic impact force is what can be expected following a mild to moderate accident to the head. When such a minor to moderate impact has the capacity to unfold laminin, it is possible that this derangement in protein structures, to some extent, may explain the complications found among the head injured patients from hockey, football and boxing activities. Minor and moderate impact may not necessarily result in cytotoxic edema but only in consisting aggregation and fragmentation of proteins (Figure 8).
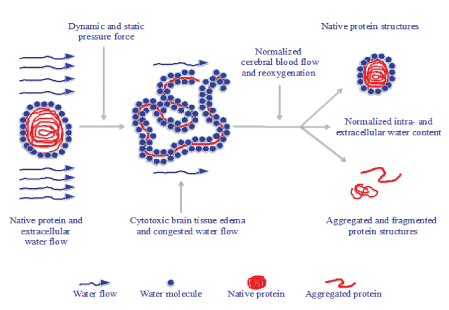
Figure 8: Schematic representation of the tentative accumulation and congestion of water molecules following protein unfolding resulting in cytotoxic intracellular edema. This condition may to some part become normalized with native protein structures after accurate clinical treatment while some of the unfolded protein structures may be aggregated and fragmented for good.
The applied low mechanical static pressure force breaks the hydrogen bonds in the outer part of the protein while higher forces are needed to unfold the more centrally located domains [19]. Thus, it is tentative to speculate about the consequences to higher speed impacts where protein unfolding, aggregation and fragmentation should become even more intense. Our earlier studies have shown that the absorbed energy following dynamic impact forces ranging from 12 to 30 m/sec or 43.2 to 108 km/hour, respectively, increases proportionally into the different tissues of the skull and brain tissue. Here rupture of both non-covalent as well as covalent bonds correlated with increased strain and intracranial pressure levels evaluated with computer simulation [5,6]. Therefore, it seems realistic to suggest that protein unfolding with a potentially increased intracellular water accumulation is caused not only by the dynamic impact force but also at moderate and severe TBI in different leisure activities. Likewise, an increased strain level in the nervous tissue following stroke due to increased mechanical static pressure force may as well be one of several plausible explanations to the cascade of their secondary consequences (Figure 8). For instance, aggregated and fragmented protein structures may result in various cognitive deficiencies. Of importance is that protein unfolding in the present study is shown already at 30 mmHg. This is about the same intracranial pressure level when patients neurological condition starts to deteriorate. With regard to the presented protein unfolding in higher experimental pressure it is possible that the protein unfolding to some extent may be proportional to the intensity of pressure force and thus similar to the hydrostatic pressure which is proportional to the depth in the sea [8]. Initiation of cytotoxic brain tissue edema by either dynamic impact force or mechanical static pressure force, the ischemic metabolism reduces the anabolic milieu while the catabolic milieu is increased. Although unfolded proteins may regain their functional status to some extent as folded ones, some of the altered proteins may continue as aggregated and fragmented structures (Figure 8). When such forces influence the head in a repetitive way often seen in leisure activities, it may be speculated that such repetitive injuries interfere with the normal protein metabolism and to some extent explain what happens to the human nervous tissue [24].
It is interesting to compare the increased hydrostatic pressure in white sharks with that of increased dynamic and static pressure force in humans with TBI and stroke in the following schematic proposal to find similarities between them (Figure 9).
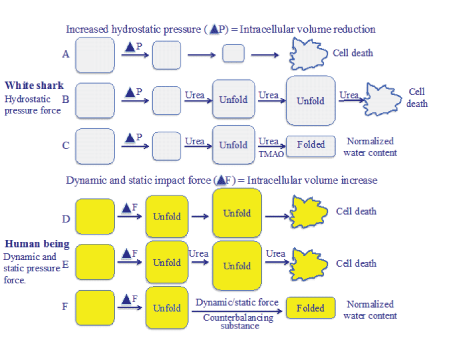
Figure 9: Schematic proposal of cellular water stress due to increased hydrostatic pressure in white sharks and with that of cellular water stress due to increased dynamic and static impact forces in human being after TBI and stroke. While white sharks balance the water accumulation with urea and TMAO to avoid water stress, human beings lack the capacity to deal with increased cellular water content.
When white sharks dive into deep sea the cells are influenced by increased hydrostatic pressure from outside with an intracellular volume reduction (Figure 9A). To prevent the deleterious water stress condition, the white sharks use urea by destabilizing the hydrogen bonds thereby unfolding the proteins aiming at counter balancing the increased hydrostatic pressure (Figure 9B). When the increased water accumulation caused by urea has regained the normal intracellular water environment, TMAO is activated to reestablish the proteins. Balancing the need for water accumulation in the cells avoids a condition of water stress (Figure 9C).
It is suggested that when dynamic impact forces and mechanical static pressure forces cause TBI and stroke, respectively, the nervous tissue responds with cellular stress defined as an intracellular volume increase resulting in cytotoxic brain tissue edema (Figure 9D). Earlier the neurosurgical treatment of cytotoxic brain tissue edema used urea. However, this substance is no longer a drug of choice since it was suggested to have a rebound effect, probably due to its osmotic effect in the disturbed blood brain barrier area. In fact, urea increased the edema (Figure 9E). A more realistic explanation to the negative clinical effect of urea is its unfolding effect on proteins [19-22]. Although the exact mechanism of action of urea is not fully understood, it is evident that it disrupts non-covalent interactions thereby denaturing the folded protein toward a more unfolded structure. However, if the clinical treatment of the human brain can find organic solutions to consider the metabolism from white sharks, the cytotoxic brain tissue edema may be avoided (Figure 9F).
It is known that mechanical force commonly occurring in the human physiology is of importance in the normal physiology of protein structures [25]. The combination of advanced computer simulation technology and laboratory results may give a more reliable atomic-level insight into biological mechanisms and will further increase the biomechanical knowledge [26]. The outcome of such investigations is of significant value in clinical neuroscience. Here the urea and TMAO metabolism in white sharks could tentatively be indicative in the search for relevant clinical treatment of TBI and stroke when it comes to prevent increased water content in the nervous tissue. Thus, if protein unfolding and water accumulation is the answer to the etiology of cytotoxic brain tissue edema, it should be easier to tailor an optimal treatment on an individual perspective and which may prevent the most severe disabled patients after TBI and stroke.
Hans von Holst presented the hypothesis, designed the experiments and drafted the manuscript. HvH and DL were responsible for the dynamic and static laboratory tests. P.P. and H.H. analyzed the results with electron microscopy. R.B.K. analyzed the results with electrophoresis. All authors provided feedback and revised the manuscript.
The authors declare no competing financial interests.
Download Provisional PDF Here
Article Type: RESEARCH ARTICLE
Citation: von Holst H, Purhonen P, Lanner D, Kumar RB, Hebert H (2018) White Shark Protein Metabolism may be a Model to Improve the Outcome of Cytotoxic Brain Tissue Edema and Cognitive Deficiency after Traumatic Brain Injury and Stroke. J Neurol Neurobiol 4(2): dx.doi.org/10.16966/2379- 7150.151
Copyright: © 2018 von Holst H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access