Introduction
Oxidative stress (OS) is referred to as a principle pathogenic process behind various neurodegenerative diseases including Parkinson and Alzheimer’s [1,2]. Generally, oxygen is required by the biological tissues to fulfill the energy requirements of the body. Nevertheless, this oxygen consumption also results in a free radical generation which possesses the capability to damage the cell. Due to the highly reactive nature of reactive oxygen species (ROS), it has a strong tendency to interact with biological molecules causing apoptosis through DNA fragmentation [3] as well as compromising the antioxidant defense system [4].
For the prevention of lipid peroxidation process and protection of oxy-radicals from the adverse influence of these free radicals, there is an “enzymatic antioxidant defense system” in the body. Different compounds have been included in the category of non-enzymatic antioxidants, for example uric acid, carotenoids, ascorbate, and α-tocopherol. However, the enzymatic defense system includes enzymes such as catalase, “superoxide dismutase” (SOD) and the “glutathionedependent enzymatic system”, and “glutathione peroxidase” (GPx). Recently much attention has been focused on Vitamin E and one of its active components alpha-tocopherol, which is considered as a medicinal remedy for a number of neurodegenerative diseases [5-7]. Vitamin E is clinically considered as a potential “free radical scavenger”, which hinders the process of oxidative stress. Moreover, it also serves as the “chain-breaking antioxidant” which precludes the proliferation of “free radical reactions” [8,9].
This research aims to assess the neuroprotective influence of alpha-tocopherol on a short-term brain slice culture derived from the frontal lobe of neonatal sheep’s brain. It was found that the pretreatment of brain slice cultures with Vitamin E for one hour followed by treatment with hydrogen peroxide (2.5mM and 5mM) suggest significantly reduced “oxidative stress” (OS) induced on the brain’s tissue. It was reflected in the increased concentration of reduced glutathione and also reduced levels of lipid peroxidation. Pretreatment of the brain slice cultures with Vitamin E promoted neuronal survival and DNA integrity, as demonstrated by the “DNA fragmentation assay.”
Materials and Methods
Chemicals
D-alpha-tocopherol (α-TP) was bought from Al Aesar (Cat. No. A17039). It was dissolved in Dimethylsulfoxide (DMSO) and used as 100 X dilutions. All other chemicals used have been bought from the “Sigma-Aldrich Co., St Louis, MO, USA.”
Brain slice culture
Neonatal sheep’s skull was obtained fresh from the local slaughterhouse. The skull was cut open and meninges were removed in sterile conditions using sterile forceps and scissors. The Frontal lobe of the brain was sliced into small pieces of 1-2mm3 and weighed under sterile conditions. The brain slices were kept in the bicarbonate buffered salt solution mimicking the composition of the cerebrospinal fluid which is commonly called Artificial Cerebrospinal Fluid (ACSF) and contains “12.4mM sodium chloride, 2.5mM potassium chloride, 1.3mM Magnesium chloride, 2mM Calcium chloride, 1.25mM dipotassium hydrogen phosphate, and 26mM Sodium hydrogen carbonate. The pH was adjusted to 7.4”. Then the solution was sterilized by autoclaving and 10mM of sterile filtered Glucose (Sigma) was added before use. The buffer was gassed with carbon dioxide and oxygen (95% O2 , 5% CO2 ) for 20-30 minutes to ensure saturation with oxygen (O2) and carbon dioxide (CO2) before using.
Treatment of brain culture
Approximately 1gm of brain slices were incubated in 10ml of the ACSF solution as described above at 37°C in a slow shaking water bath for each incubation type. Following are the different conditions of incubation for the brain slice cultures. The brain slice cultures (BSC) were treated as follows.
Group 1: BSC which was incubated without any treatments.
Group 2: BSC which was incubated with DMSO alone.
Group 3: BSC which was incubated with Vitamin E alone.
Group 4: BSC which was incubated for one hour without any treatment with vitamin E, followed by treatment with 2.5mM H2O2 .
Group 5: BSC which was pre-incubated with 10 µM of alphatocopherol for one hour followed by the treatment with 2.5mM H2O2 for an additional one hour.
Group 6: BSC which was incubated for one hour without the treatment followed by treatment with 5mM H2O2 for one hour.
Group 7: BSC which was incubated in the presence of 10 µM Vitamin E for one hour followed by treatment with 5mM H2O2 .
Preparation of brain tissue for biochemical Assay
After incubation, the conditioned medium was removed from the brain tissue slices and kept for reduced glutathione assay. The brain tissue was cleaned with sterile cold PBS that was subsequently homogenized in 5ml of cold sterile PBS. About 1ml of brain homogenate was used for each biochemical assay.
DNA fragmentation and quantitation Assay
The degree of “DNA fragmentation” was assessed through the method described by Taylor [10]. Brain tissue homogenate was lysed with an equal volume of lysing buffer, “5mM Tris/ HCl pH 8, 20mM EDTA, and 0.5% triton X-100”, and this lysate was centrifuged for the isolation of fragmented chromatin and intact chromatin. A concentration of “12.5% trichroacetic acid” (TCA) was used to precipitate supernatant and pellet, then the precipitated DNA was treated for 10 min at a temperature of 90°C in 1ml of 5% TCA. Furthermore, a reaction involving diphenylamine was conducted for quantitative analysis overnight at room temperature. The optical density was measured at 600nm against blank. The fragmentation percentage was analyzed as “the ratio of DNA in the supernatant to the total DNA (supernatant plus pellet).”
DNA extraction and electrophoresis
The influence of H2O2 on fragmentation of DNA has been analyzed through gel electrophoresis method, as demonstrated by Lee et al. [11]. The tissue homogenate was re-suspended in a lysis buffer (10mM Tris-KCl pH 8.0, 10mM NaCl, 10mM EDTA, 100 µg/ml proteinase K, and 1% SDS). Phenol/chloroform was used to extract the lysate with the ratio of 1:1, v/v, while ethanol was used to precipitate the DNA fragments. In the buffer, DNA pellets were determined as “10mM Tris-HCl, 1mM EDTA, and pH 8.0”. Then, the buffer loaded DNA samples were introduced into 1.8% agarose gel. Afterwards, the DNA was photographed followed by the visualization through ethidium bromide.
Reduced glutathione assay
This assay was carried out through the procedure of Beutler et al. [12]. In this assay, about 1 ml of brain homogenate and 1ml of conditioned medium were used. Then, 1.5 ml of “double distilled water” (DDW) was introduced to the tissue homogenate and conditioned medium, which was then preserved in 0.6 ml of precipitating chemical containing “1.67 g of glacial metaphosphoric acid, 0.2 g of EDTA and 30.0 g of NaCl” made up to 100 ml with DDW. The mixture was placed in the centrifugation machine for 10 minutes at a speed of 1200xg. To “0.3 ml of supernatant, 2 ml of Na2HPO4 (0.3 M) and 0.25 ml of 5, 5’ dithio-bis-2-nitrobenzoic acid (DNTB), and 0.4 % in 1% sodium citrate” were added, and volume was made up to 3 ml with DDW. The OD was measured against blank at 412 nm, while the value was expressed as µg of reduced glutathione per gm of tissue.
Lipid peroxidation assay
“Lipid peroxidation” (LP) was calculated using the procedure of Garcia et al. [13], using the TBARS (thiobarbiturate reactive substances) assay. About 1 ml of homogenate of the brain was placed in an incubator for one hour at 37°C. Approximately 1.5 ml of 20% TCA was added and centrifuged at 600 xg for 10 minutes, and 1 ml of newly arranged “Thiobarbituric acid” (TBA) 0.67% was added to 1ml of the supernatant, and the reaction mixture was heated in a boiling water bath for 10 minutes. Absorbance was recorded at 535nm by the help of comparison with a blank reagent. The values were expressed as mM of malonaldehyde formed hour/gm of the tissue.
Statistical analysis
Values were expressed as mean ± S.D. The data were represented statistically in the form of numbers, standard deviation (SD), and mean. The contrast among various groups was done using an independent sample T-Test using Microsoft Excel program 2010 for comparing two groups. A “probability value” (p-value) of <0.05 was referred to be significant. For the statistical analyses, SPSS program (v22.0.0.0) was used.
Results
Effect of alpha-tocopherol on H2O2 induced cytotoxicity
DNA fragmentation assay using diphenylamine showed that the percentage DNA fragmentation was significantly reduced when the brain slices were pretreated and co-incubated with vitamin E at both 0.25mM and 0.5mM H2O2 concentrations (Figure 1). The %DNA fragmentation was reduced to 5% with the pretreatment of brain cultures with vitamin E as compared to the control cultures. The % DNA fragmentation at 2.5mM was reduced to about 4% when the cultures were treated with 2.5mM of hydrogen peroxide in the presence and absence of alpha-tocopherol. The %DNA fragmentation at 5mM was reduced to 12% when the pretreated and control cultures were compared. This study was further supported by agarose gel electrophoresis in which the genomic DNA was extracted from the samples and subjected to 1.8% agarose gel. This showed that there is extensive fragmentation of DNA with the treatment of H2O2 (Figure 2); however, the presence of Vitamin E protected DNA from undergoing fragmentation as shown in figure 2.
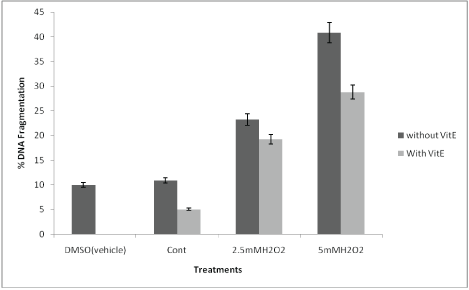
Figure 1: Histogram of %DNA fragmentation at various culture conditions: Percentage DNA fragmentation was calculated by diphenyl amine assay method as described in materials and method section. The DNA damage in brain tissues treated with DMSO (vehicle) alone, the brain cultures treated with Vitamin E alone, the control brain culture without any treatments. Brain cultures pretreated with 10 µM alpha tocopherol alone and pre-treatment with 10 µM alpha tocopherol followed by induced oxidative stress by 2.5mM H2O2 and 5mM H2O2.
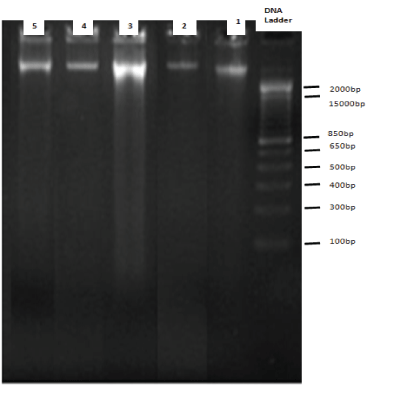
Figure 2: 1.8% agarose gel electrophoresis: Genomic DNA was isolated from different treatment Groups of Brain Tissues and run on 1.8% agarose gel. DNA bands were visualized by ethidium bromide staining and photographed. Band 1 is the genomic DNA isolated from Group 1, Band 2 is the genomic DNA isolated from Group 2 (With alpha tocopherol only), Band 3 is genomic DNA isolated from Group 5 (with 5mM H2O2 treatment alone), Band 4 and 5 is genomic DNA isolated from Group 6 and 7 (pre-treatment with alpha tocopherol followed by 2.5mM, 5mM H2O2 ) respectively.
In all of these experiments, the effect of DMSO alone was studied as well as it was used as a vehicle for the treatments. According to the experiments performed in this study, DMSO alone did not alleviate the level of oxidative stress or the amount of DNA fragmentation induced by H2O2 . The neuroprotective effect of vitamin E on Hydrogen peroxide-induced oxidative stress on the lipid peroxidation level was also investigated (Table 1). The lipid peroxidation level was calculated by the mM of malonaldehyde formed per hour per gram.
| |
TBARS (mM of Malondehyde formed/ hour/gm (Tissues) |
Reduced Glutathione (µg GSH/gm) |
| Conditioned Medium |
Tissue |
| Group 1 (Control) |
7.526 ± 0.64 |
2.32 ± 0.09 |
1.73 ± 0.06 |
| Group 2 (With DMSO) |
6.255 ± 0.07 |
2.24 ± 0.07 |
1.64 ± 0.03 |
| Group 3 (alpha-tocopherol) |
3.782 ± 0.08* |
2.66 ± 0.01 |
1.865 ± 0.02 |
| Group 4 (2.5mM H2O2
) |
9.519 ± 0.03 |
0.1595 ± 0.07 |
0.449 ± 0.06 |
| Group 5 (2.5mM H2O2 ) +Alpha-tocopherol) |
5.301 ± 0.37* |
1.392 ± 0.06* |
1.116 ± 0.10* |
| Group6 (5mM H2O2 ) |
10.183 ± 0.05 |
0.2285 ± 0.05 |
0.409 ± 0.03 |
| Group 7 (5mM H2O2 ) +Alpha-tocopherol) |
4.375 ± 0.09* |
0.6165 ± 0.05* |
1.902 ± 0.05* |
Table 1: The effect on oxidative stress (reduced GSH) and lipid peroxidation levels on pre-treatment of neonatal brain slice cultures with alpha tocopherol,
in the presence and absence of induced oxidative stress by 2.5mM H2O2 and 5mM H2O2. The values were expressed as the mean of three independent
experiments and ± SD were calculated. (*) represent the values are significantly (p˂ 0.05) different in the cultures with induced oxidative stress by
hydrogen peroxide in the presence and pre-treatment with Alpha tocopherol, when compared to the hydrogen peroxide treated cultures alone.
Treatment with vitamin E alone showed a reduced amount of lipid peroxidation when compared with the control cultures. However, the lipid peroxidation measured in the control cultures as the amount of malonaldehyde formed per hour was not significantly (p<0.05) different from 2.5mM and 5mM hydrogen peroxide-treated cultures for one hour. The lipid peroxidation level was significantly reduced (p<0.05) with the pretreatments of the brain slice cultures with Vitamin E and then exposed to H2O2 treatments when compared to the hydrogen peroxide-treated cultures alone both at 2.5mM and 5mM concentrations.
Another marker of oxidative stress i.e., reduced glutathione levels (GSH) were also investigated in this study. Reduced glutathione levels were measured in the conditioned medium as well as present intracellularly. Reduced glutathione levels were significantly increased in the brain cultures that were pretreated and co-incubated with Vitamin E and H2O2 compared to the H2O2 treated cultures alone (Table 1). The levels of reduced GSH intracellularly were increased eight-fold in the cultures pretreated with vitamin E and then co-incubated with 2.5mM H2O2 , when compared to 2.5mM hydrogen peroxide-treated cultures alone. The levels of reduced glutathione in the brain tissues n were also shown to have increased to three-fold with the pretreatments of brain Tissues with Vitamin E and subsequently subjected to 5mM hydrogen peroxide when compared to the Group 6 (5mM H2O2 alone). A slight increase in reduced GSH levels was observed in Group 6 (5mM H2O2 treated brain tissues) when compared to Group 4 (0.25mM H2O2 ), which could be due to the fact that under increased OS brain Tissue secrete more GSH into the medium to combat increased OS thus depleting intracellular GSH. However, with the pretreatments with vitamin E to the brain cultures, GSH levels were significantly increased (p< 0.05) even when these cultures were induced OS with 2.5mM and 5mM H2O2 .
Discussion
In this study, the short-term brain slice culture was used to study the therapeutic potential of Vitamin E. Brain slice culture has a unique advantage over other “in vitro and in vivo experimental models” as this maintains the neuronal activity with complete functional “local synaptic circuitry” and permits direct treatments through pharmacological agents that are capable to modulate the response [14-16]. The alpha-tocopherol has the ability to cross the “blood-brain barrier” (BBB) and protecting the neuronal cells through the “oxidative stress” (OS), which can be considered as a principle contributing factor in various neurodegenerative diseases, as elucidated in a number of studies [5].
Most studies that were previously conducted, have examined the influence of Vitamin E on the OS induced by co-incubation of Vitamin E with the agonist. In contrast, this study examined a hypothetical function of vitamin E for neuroprotection, so brain slice cultures remained pre-incubated with vitamin E before treating them with H2O2 inducing oxidative stress. This is of particular interest as the neuroprotective property of vitamin E is not only primarily attributed to its inherent antioxidant action but may also be mediated through other cellular functions. The concentration chosen for the treatment is 10 µM which has been shown to be the optimum concentration in other in vitro studies [17].
H2O2 is considered as a principle reactive oxygen species, also known as ROS that is produced by the process of redox reaction and acts in “intracellular signaling cascade” as a messenger [18]. This compound is also used as the initiator of OS in the in vitro models, as it causes “mitochondrial membrane dysfunction”, lipid peroxidation, and immense damage in the DNA structure [19].
Furthermore, the dietary antioxidants are also significant for the prevention of toxic effect induced by “endogenous reactive oxygen species”, while many studies assert that vitamin E possesses a potential role against OS. In addition, the vitamin E deficiency may elevate the vulnerability to OS-induced neuronal toxicity. A number of researches have investigated the vitamin E supplementation function to reduce DNA damage and OS [20-22]. Vitamin E is composed of alpha- tocopherol, betatocopherol, gamma- tocopherol, and delta-tocopherol isomers. However, in this research, alpha-tocopherol was selected for investigation, which is one of the isomers of Vitamin E. Alpha-tocopherol is generally considered as a powerful antioxidant because of its high bioavailability and bioactivity. Structurally, alpha-tocopherol has electron-donating methyl groups attached to its chromanol ring, which act to inhibit lipid peroxidation [23].
As far as the “free radical scavenging properties” of alphatocopherol are concerned, the current research has been conducted to assess the vitamin E neuroprotective potential in the neonatal sheep brain slice culture. This research has explored the neuroprotection potential of Vitamin E in terms of its effect on neuronal cell viability using the DNA fragmentation assay by diphenylamine method. In measuring apoptosis, “diphenylamine assay” is considered as a significant tool to determine the fragmentation percentage of known DNA amounts in oligosomal-sized fragments. One more benefit is that this diphenylamine assay is the apoptotic analysis of DNA fragmentation in both shed and adherent cells prior to the experimentation or treatment. This assay was first described in 1930’s but modified considerably by other researchers [10,24]. These modifications have resulted in an improved susceptibility of almost five folds by the addition of acetaldehyde and sulfuric acid, as well as by permitting the “colorimetric reaction” to take place at room temperature overnight. Such alterations were shown to reduce an interface through certain substances which were considered initially as a drawback in an originally labeled method, which in turn improved the sensitivity of assay. Meanwhile, a reaction of diphenylamine took place, emphasizing the benefits of deoxyribose and purines bonding that were immensely labile. As soon as the bonds were broken, there was the liberation of inorganic phosphates throughout the DNA to give off the substrate product that was assessed through this reaction. This study was further supported by DNA fragmentation assay on an agarose gel, which clearly showed the integrity and stability of the genomic DNA derived from Vitamin E treated cultures although they induced oxidative stress with H2O2 as compared to the cultures that were exposed to H2O2 alone.
Such deleterious effect of ROS in this “Lipid peroxidation” can be significantly implemented to treat several acute as well as chronic brain disorders [25,26]. The most common and prevalent “lipid peroxidation assay” is regarded as TBARS assay that involves the measurement of end products’ reactivity as a result of lipid peroxidation, i.e., MDA that reacts with the TBA to give off red adduct which can be quantified spectrophotometrically. In this research, “lipid peroxidation” was found to have decreased in the conditions where Vitamin was present. Vitamin E has been suggested as primary antioxidant reacting with peroxyl radicals produced by lipid peroxidation in other studies [27].
Therefore, it can be assumed from the present findings that there is a reaction between the oxygen molecule and α-tocopherol prior to the development of OH molecule. However, thorough destruction of intracellular oxygen is considered to represent a significant neuroprotective action mechanism including alpha-tocopherol in the striatal cultures among rats.
The neuroprotective effect of alpha-tocopherol is by both antioxidant and non-antioxidant pathways in vivo [9]. It has been observed that hydrogen peroxide treatments can cause depletion of glutathione levels and accumulation of intracellular peroxidases. However, the reduction of H2O2 cytotoxicity through pretreatments with Vitamin E suggests that neuroprotection mediation is through the cellular effect which may include modulation of glutathione metabolism.
Overall, this study showed that pretreatment of brain slice cultures with Vitamin E may substantially increase a viability of neuronal cell by increasing the antioxidants enzymes such as the amount of reduced GSH and remarkably reduce the “Lipid peroxidation levels.”
Conclusion
Apoptosis is an oxidative stress play an important role in the pathogenesis of a number of neurodegenerative disorders. A neuroprotective effect of alpha-tocopherol on H2O2 -induced OS in short-term neonatal sheep’s brain tissue culture was investigated. The results showed that the DNA fragmentation and apoptosis is caused by H2O2 treatments to the brain cultures were attenuated significantly in the occurrence of vitamin E. Moreover, these findings also suggest that the levels of “lipid peroxidation” were significantly reduced in the presence of Vitamin E, suggesting a possible neuroprotective role of vitamin E against oxidative stress. The Glutathione levels were also increased significantly; thereby, mitigating hydrogen peroxideinduced oxidative stress. This study suggests that α-tocopherol may be directly involved in neuronal survival by reducing the oxidative stress in vitro.
Acknowledgement
We are deeply grateful to Department of Biochemistry, College of Science, King Saud University for facilitating this research. We also acknowledge the support of deanship of female research Centre.
Declaration of Interest
There is no conflict of interest to declare.



