Introduction
Mild cognitive impairment (MCI) is the early clinical stage of
Alzheimer’s disease (AD) and it’s characterized by impairment in learning
and memory [1,2]. Nowadays, more than 20% people over 80 years old of
age are affected by AD. Epidemiological data predict that over 35 millions
of people will be affected by 2050 over world [3], which will cause heavy
social and economic burden. So far, limited benefits have been obtained
from available therapeutic agents to slow down this disease progression.
Menopause women, as the decline of estrogen level, are susceptible to
cognitive impairment [4,5]. Ovariectomy in female rodents is a widely
recognized model to mimic postmenopausal pathophysiological changes
in humans [6,7]. Our previous results [8] and other experimental data
[9] demonstrate that decreased estrogen levels in ovariectomized rodents
are associated with decreased brain activity overall and poor memory,
especially hippocampus-dependent learning [10]. As researchers Orawan
et al. once reported that ovariectomized mice not only decreased serum
17-estradiol level and uterine weight, but also impaired object recognition
performance in the novel object recognition test and spatial cognitive
performance in the Y-maze test and the water maze test [11].
Early growth response 1 (Egr1) belongs to the zinc finger family
of transcriptional factors [12,13], which participates in a variety of
mechanisms mediating growth, proliferation, differentiation and
apoptosis [14-16]. Recently, researches have shown that the Egr1 changes
attribute to the progression of memory deficit [17,18]. Gersten et al.
studied that Egr1, as a key molecule in hippocampus-related learning and
memory is down-regulated in simian immunodeficiency virus-infected
hippocampus, leading to deficits in cognition [19]. Interestingly, our
recent study showed that Egr1 messenger RNA (mRNA) and protein
level were elevated in ovariectomized mice, which were linked to their
cognitive impairment [20].
mTOR belongs to a family of specific serine/threonine protein kinases.
Protein mTOR exists in two mTOR protein complexes mTORC1 and
mTORC2 with various sensitivity to the inhibitory effect of rapamycin
[21]. p70S6K (the serine/threonine kinase ribosomal protein) is one of the
major downstream targets regulated by mTOR activity [22]. In recent years,
more and more studies have shown that the mTOR signaling pathway is
closely related with cognition deficiency [23,24]. Besides, overwhelming
evidence showed that the elevated activity of mTOR signaling pathway
has been found in AD patients [25,26]. Caccamo et al. have reported that
suppressing mTOR signaling could rescue the memory deficits in Tg2576
mice that are widely used animal model of AD [27]. Mechanistically,
the reduction in mTOR signaling could restore the hippocampal gene
expression signature [28]. These results implicate that hyperactive mTOR
signaling may represent a molecular pathway by which aging contributes
to the development of AD.
Interestingly, the abnormal expression of Egr1 and mTOR/p70S6K
signaling respectively has relationship with cognition deficits. Whether
there is potential association between the over-expression of Egr1 and
mTOR/p70S6K signaling activity remains to be illuminated. Therefore,
in the present study, we investigated the changes of the mTOR/p70S6K
signaling pathway and cognition function in OVX mice, and further
clarified the potential relationship between Egr1 expression and the
mTOR/p70S6K pathway. Through our study, we hope to provide clues for
the prevention and treatment of cognitive impairment of postmenopausal
women in the future clinical practice.
Materials and Methods
Animals and surgery
Female ICR mice (Medical Animal Center of Nanjing Medical
University, Jiangsu Province, China), weighed 25-30 g (8-12 weeks) at the
beginning of the experiment, were used throughout the study. All animals
were housed under a standard light/dark cycle at a mean (SEM) constant
temperature of 23°C (±1° C). Mice (n=50) were anesthetized with chloral
hydrate (500 mg/kg IP) and randomly divided into two groups to undergo
either bilateral ovariectomy (n=25) or sham operation (n=25) by the
ventral approach using sterile surgical techniques. Visual inspection
corroborated the complete resection of the ovaries. Before and after all
procedures, five mice were housed in a plastic hanging cage and permitted
free access to food and tap water. The study was approved by the Animal
and Human Ethics Board of the First Affiliated Hospital, Nanjing Medical
University. All efforts were made to minimize animal suffering and to
reduce the number of animals used.
Morris water maze test
Morris water maze (MWM) test was performed to test learning and
memory in mice. It was divided into four quadrants: southeast, southwest,
northeast, and northwest, with a 10 cm black circular escape platform
placed at a fixed location, approximately 2 cm under the water surface.
Exposed at 25°C room temperature, the water was opaque and the pool
was coated with black non-toxic tempura paint. Mice were trained to find
the hidden platform for 5 consecutive days, four training trials per day. If
the mouse reached the platform within 60 s, it was allowed to stay on it
for 10s; otherwise, it was gently guided to the platform and remains on it
for 10s. Time taken to reach the platform (escape latency) and spent in the
target quadrant was recorded by a video tracking system during probe tests.
Drug administration
An intracerebroventricular injection was performed through a
stereotaxic apparatus. Mice were anesthetized with 2% chloral hydrate (20
ml/kg) and placed in a stereotactic device (Kopf Instruments, Tujunga,
CA). The injection site was confirmed in preliminary experiments by
injecting Indian ink. A 26-gauge single guide cannula (Plastics One,
Roanoke, VA) was implanted into the right lateral ventricle (0.3 mm
posterior, 1.0 mm lateral, and 2.5 mm ventral to bregma). After surgery, a
28-gauge dummy cannula (Plastics One, Roanoke, VA) was inserted into
each guide cannula. Neither insertion of the needle nor injection of the
saline had a significant influence on survival, and behavioral responses or
cognitive functions.
Rapamycin was dissolved in dimethylsulfoxide (DMSO, Sigma, St
Louis, MO, USA) and then in saline solution, final concentration being
20 mg/kg. DMSO was used at a final concentration of 2% as vehicle
control. The drugs were injected with a stepper-motorized microsyringe
(Stoelting, Wood Dale, IL, USA) at a rate of 0.5 ml/min (final volume 0.6
µl/mouse). Rapamycin (1.0 mg/kg) or equal volume vehicle control was
delivered gradually over the course of 2 min.
Cell culture and treatment
SH-SY5Y neuroblastoma cell line (ATCC, Shanghai, China) is a very
popular cell model to clarify the molecular mechanisms of Alzheimer
disease in many researches [29,30]. SH-SY5Y Cells were cultivated
in Dulbecco’s modified Eagle’s medium/F12 supplemented with 10%
heat-inactivated fetal bovine serum (Tianhang, Hangzhou, China) in
a humidified atmosphere containing 5% CO2 at 37˚C. To evaluate the
relationship between Egr1 and mTOR/p70S6K signaling pathway, we
transfected SH-SY5Y cells with Egr1-overexpressing plasmid (GV141-
Egr1 plasmid) , GV141-Egr1 control (vector), Egr1 siRNA and si-Egr1
control (si-Egr1 NC) (Genechem, Shanghai, China) using Lipofectamine
3000 (Invitrogen, Carlsbad, CA). Besides, the mTOR inhibitor rapamycin
(4 µM) was applied to the cells to further conform the results. Cells were
collected for total RNA isolation or protein purification at 48 hours after
transfection or rapamycin treatment.
RNA extraction and quantitative real-time PCR
Homogenization of the fresh hippocampus tissues and isolation of total
RNA were performed according to the manufacturer’s instructions using
a Trizol-based commercial kit (Takara Shuzo Co. Ltd., Kyoto, Japan). The
purity of each RNA sample was determined by the absorbance ratio at
260 and 280 nm. The integrity of RNA preparations was evaluated by
electrophoresis on a 1.2% (w/v) agarose gel containing 0.005% (v/v) a
nucleic acid dye, Goldview (Shanghai SaiBaiSheng, Shanghai, China).
The extracted RNA, containing ribosomal 28S and 18S RNA with a ratio
of absorbance intensity 1.0–1.5 was used for qRT-PCR. In the real-time
PCR reaction, cDNAs were used as templates for amplification to quantify
the mRNA levels of target genes by using Quantitect SYBR Green PCR
kits (Takara Shuzo Co Ltd, Kyoto, Japan). Glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) or U6 was used as an internal control for
sample loading and normalization. The comparative Ct (threshold cycle)
method with arithmetic formulas (2-ΔΔCt) was used to determine the
relative amount of mRNA.
Western blot analysis
Total protein samples were homogenized in RIPA buffer containing
protease inhibitors and phosphatase inhibitors. Proteins from all
extractions were quantified using a BCA Protein Assay Kit (KC-430; Kang
Chen). The protein samples were separated by 8% sodium dodecyl sufatepolyacrylamide
gel electrophoresis and transferred to a polyvinylidene
difluoride membrane (Millipore, Billerica, MA). The membranes were
blocked with 5% nonfat milk or 5% bovine serum albumin in Tris-buffered
saline+1% Tween 20(TBST) for 1 hour at 37˚C. Then membranes were
incubated with the appropriate primary antibodies: rabbit anti phosphomTOR
(p-mTOR) antibody (1:1000, Ab109268; Abcam, London, UK),
rabbit anti-mTOR antibody (1:1000, Ab87540; Abcam), rabbit anti
phospho-p70S6K (p-p70S6K) antibody (1:1000, Ab109393; Abcam),
rabbit anti-p70S6K antibody (1:1000, Ab32359; Abcam), rabbit anti-Egr1
antibody (1:5000, ab194357; Abcam), and rabbit anti-tubulin antibody
(1:5000, ab176560; Abcam) overnight at 4°C. After five washes with TBST
for 10 minutes at 37°C, the membranes were incubated with horseradish
peroxidase-conjugated secondary antibody (Beijing ZhongShan, Beijing,
China) for 1 hour at 37°C. Bands were detected using an ECL detection kit
(Amercontrol Biosciences,London, UK).
Immunofluorescence and confocal imaging
To evaluate intracellular expression levels of egr1 and mTOR by using
immunofluorescence and confocal microscopy, SH-SY5Y cells were
cultured on poly-d-lysine coated coverslips for 24 h. Cells plated on
coverslips were then fixed in 4% paraformaldehyde in phosphate-buffered
saline (PBS) for 20 min, washed in PBS one time, permeabilized in 0.1%
Triton X-100, and blocked in 1% BSA, 50 mM glycine and 2% normal
serum. The primary antibodies against the following proteins were used:
rabbit anti-mTOR antibody (1:100), rabbit anti-Egr1 antibody (1:100),
for overnight at 4°C. On the next day, coverslips were washed with PBS
and then incubated with the FITC (fluorescein isothiocyanate) marked
secondary antibodies and applied for 1 h at room temperature. To stain the
nuclei,1 μg/ml(w/v) 4′, 6-diamidino-2-phenylindole (DAPI, Sigma, USA)
was added for 5 min. Confocal immunofluorescence images (1024×1024
pixels) were acquired on the Olympus Fluoview 1000 laser scanning
confocal microscope using a 20x and 40x objective with numerical
aperture 1.42.
Electrophysiological analysis
Brains were quickly removed and placed in ice-cold oxygenated
artificial cerebrospinal fluid (ACSF) consisting of (in mM) 124NaCl,
2CaCl2
, 4.5KCl, 1.0 MgCl2
, 26NaHCO3
, 1.2NaH2
PO4
, and 10D-glucose
and adjusted to pH7.4 by bubbling with 95% O2
/5% CO2
mixture. Coronal
brain slices (400-mm-thick) were cut using a vibrating microtome
(Microslicer DTK 1500, Dousaka EM Co, Kyoto, Japan) in ice-cold
oxygenated (95% O2
/5%CO2
) ACSF.
For recording, the slices were transferred to a chamber continuously
perfused with oxygenated ACSF (2ml/min) maintained at 30˚C.
Stimulation-evoked population spike (PS) were recorded from the BLA
by a glass micropipettes filled with 2 M NaCl (4-5 MΩ) connected to
an Axoclamp2B amplifier (Axon Instruments, Foster City, CA, USA).
PS response was sampled using pCLAMP software (Axon Instruments,
Foster City, CA, USA). Input-output (I/O) curve was built by plotting
excitatory post-synaptic potential (EPSP) slopes against delivering
stimulation intensities from 0.1 mA to 1.1 mA that ranged from subthreshold
intensity for elicitation of a PS to those eliciting maximal
responses. To induce long-term change, a single train of high frequency
stimulation (HFS) with 50% of maximal stimulus strength at 100 Hz for
1 s duration was delivered. To evaluate long-term potentiation (LTP), the
same recording as that pre-HFS continued for 60 min post-HFS and the
data were expressed as the percentage of the mean pre-HFS value. The
successful LTP induction requires the increase of PS amplitude post-HFS
during the stable phase (>30 min post-HFS) exceeds a minimum of 20%.
Statistical analysis
The data were shown as the mean ± standard deviation and the results
were analyzed using one-way ANOVA test together with the Scheffe’
multiple-range test by the SPSS statistical package 20.0 (SPSS Inc., Chicago,
IL, USA). The one-way ANOVA test was used to evaluate generally the
difference among groups and the Scheffe’ multiple-range test was used to
compare the two groups. P<0.05 was considered as statistical significance.
Results
Changes of cognitive behavior in ovariectomized 12-week mice
During the training, all mice spent less time to find the hidden
platform day by day, but the 12-week-old OVX mice significantly show
a longer escape latency than the age-matched sham-op mice in the last
day of training trials (P<0.05, n=25; Figure 1A). Compared with Sham
mice, the 12-week-old OVX mice spent less time in the quadrant that the
hidden platform located before (P<0.01; Figure 1B). As shown in Figure
1C, the exploring trait typical swimming patterns in Sham mice were
characterized by an obviously longer distance in target quadrant, whereas
concentric swimming paths representing reduced platform crossings were
found in OVX mice.
Influence of OVX on hippocampal LTP induction
To explore the effects of ovariectomy on neurons, we investigated the
electrophysiological changes of perforant path-granule cell synapses. As
shown in Figure 1D, the EPSP slopes in ovariectomized 12 week-mice were
consistently smaller than that in the age-matched Sham mice at the stimulation
of 0.1-1.1 mA (P<0.05; n=6). HFS can induce a significant increased EPSP
in perforant path-granule cell synapses lasting for at least 60 minutes (which
is named as LTP) in the hippocampus of Sham mice. However, similar HFS
cannot result in LTP induction in ovariectomized mice (Figure 1E).
Expression of Egr1 and mTOR pathway in mouse hippocampus
The expression levels of Egr1 mRNA and Egr1 protein in the
hippocampus were significantly increased in 12-week-old OVX mice
compared with the Sham group (P<0.01; Figure 2C). Notably, we found
that mRNA level of mTOR/p70S6K signaling pathway also upregulated
in OVX mice. As shown in Figures 2A and 2B, the expression of mTOR
and p70S6K mRNA were 1.45 fold and 1.38 fold higher respectively in
the hippocampus at 12 weeks after ovariectomy compared with shamoperated
mice (P<0.05). Besides, our results suggest that not only total
protein of mTOR and p70S6K upregulated in OVX mice, phospho-mTOR
at Ser2448 and phospho-p70S6K at Ser371 protein were significantly
higher than Sham mice (P<0.05; Figure 2G).
To explore the relationship between Egr1 and mTOR signaling pathway,
OVX mice after MWM test were treated with the mTOR inhibitor
rapamycin (1.0 mg/kg) for a week via intra-cerebroventricular injection
(i.c.v). In comparison to vehicle control, the rapamycin treatment
negatively regulated Egr1 mRNA expression (P<0.01; Figure 2F), as well
as mTOR signaling pathway (P<0.01; Figures 2D and 2E). Results of
protein changes were consistent with mRNA analysis (P<0.05; Figure 2H).
Association of Egr1 expression and mTOR signaling pathway in
SH-SY5Y cells
Through immunofluorescence and confocal microscopy, the
subcellular localization of Egr1 and mTOR in SH-SY5Y cells were
evaluated. The subcellular localization of Egr1 was mainly in perinuclear
cytoplasm and nuclei (Figure 3A), while mTOR was mainly observed in
perinuclear cytoplasm (Figure 3B). Next, we transfected cells with the siEgr1
fragment or the Egr1 overexpressing plasmid to explore the effect
of Egr1 on mTOR/p70S6K expression. 48 hours after transfection with
Egr1-overexpressing plasmid or si-Egr1 fragment, mTOR and p70S6K
expression in SH-SY5Y cells were not changed compared with the control
group, including the mRNA and protein levels (P>0.05; Figures 4A-4H).
To further confirm the association of Egr1 and mTOR/p70S6K, cells
were finally treated with rapamycin (4 µM) for 48 hours. Consistent with
the above results in hippocampus of OVX mice, rapamycin treatment
negatively regulated Egr1 mRNA expression (P<0.01; Figure 5C), as well
as mTOR/p70S6K signaling pathway (P<0.01, P<0.05; Figures 5A and
5B). There was similar results in protein levels after rapamycin treatment
(P<0.05; Fiure 5D).
Discussion
In this study, we confirmed that learning and memory may be impaired
by ovariectomy at 12 weeks mice, including the increased escape latency,
reduced time in target platform quadrant through MWM test, which is
similar to our previous results [31]. Moreover, we found that there was
remarkable difference of electrophysiological examination in OVX and
Sham mice, in that EPSP slopes in ovariectomized mice were consistently
smaller than in the Sham mice and the deficit of LTP induction in OVX
was occurred. Generally, EPSP slopes and LTP induction were used to
evaluate synaptic plasticity of neurons, which is closely related with
cognition function [32,33]. According to many studies, estrogen can
dramatically increase hippocampal dendritic spine and synapse density
and its protective function in cognition has been well discovered in recent
years [34,35]. Thus, estrogen absence in mice 12 weeks after ovariectomy
could impair synaptic plasticity in our research, and finally leading to
cognitive decline. These results were supported by some other studies
concerning the electrophysiological changes after reducing estrogen level
in mammalian model [36,37].
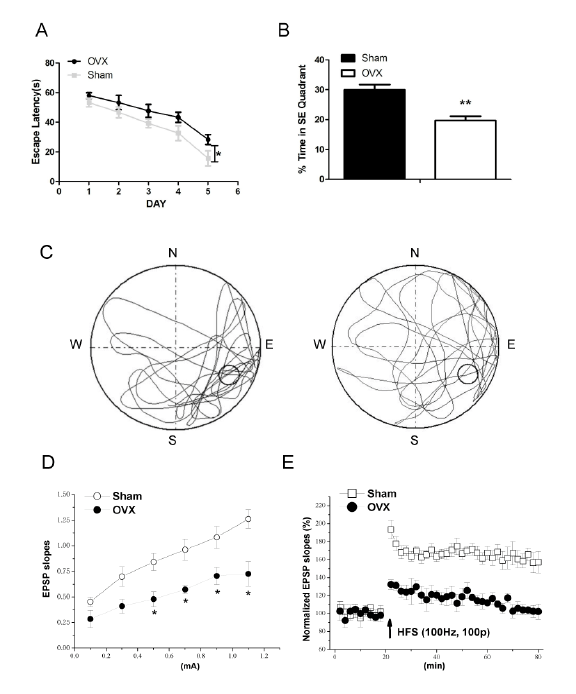
Figure 1: Changes in cognitive function at 12 weeks after ovariectomy
A: Comparison of escape latency in finding the platform in three other nontarget quadrants between ovariectomized (OVX) and sham-operated
(Sham) mice. B: Time spent swimming in the target quadrant in the probe trial during 5 days of training in Morris water maze (MWM) for each group.
C: Representative path tracings of the probe test on day 6 of MWM for each group. D: Input-output (I/O) curve. EPSP slope was evoked by perforant
path-stimuli with a current from 0.1 to 1.1 mA. Each point represents group mean value (SEM) of EPSP slope. E: Changes of path-granule cell
synaptic transmission in OVX and Sham mice slices with induction of LTP by 100 Hz-CS. *P<0.05, **P<0.01.
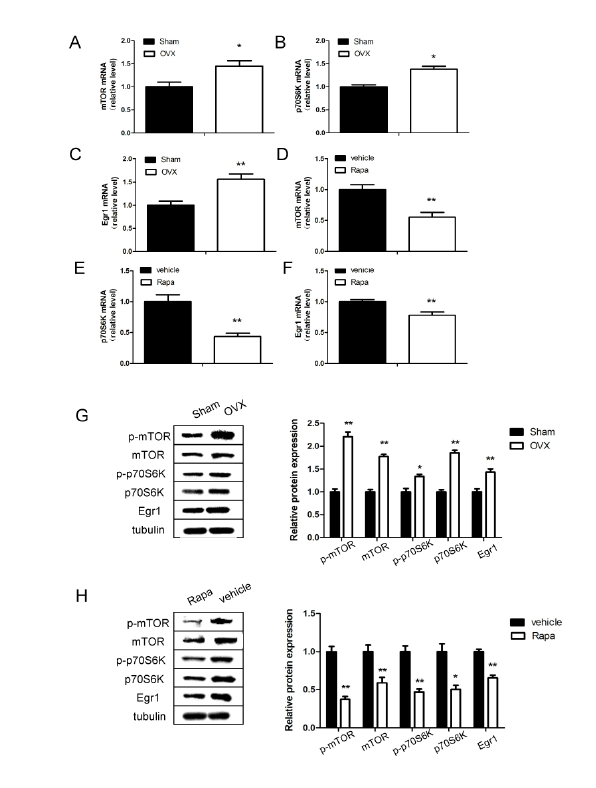
Figure 2: Expression of Egr1, mTOR and p70S6K in the hippocampus of ovariectomized (OVX) and sham-operated (Sham) mice
Quantitative reverse transcription polymerase chain reaction analysis of messenger RNA levels of mTOR (A and D), p70S6K (B and E) and Egr1 (C
and F) in ovariectomized and rapamycin treatment mice respectively. G: Western blot analysis of protein levels of Egr1, mTOR and p70S6K 12 weeks
after ovariectomy. H: Relative protein levels of Egr1, mTOR and p70S6K after rapamycin treatment for a week. *P<0.05, **P<0.01.
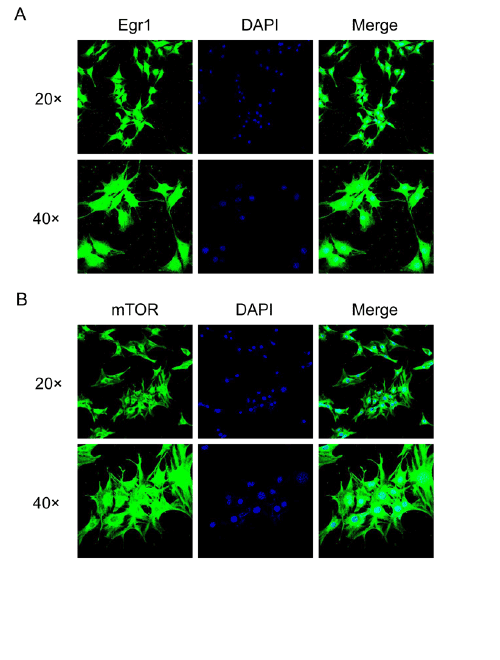
Figure 3: Confocal immunoflorescence microscopy of subcellular localization of Egr1 and mTOR in SH-SY5Y cells.
A: subcellular localization of Egr1 protein. B: subcellular localization of mTOR protein. Cells were seeded onto coverslips and stained respectively
with Egr1 and mTOR antibodies, then the FITC (green) marked secondary antibodies. The nuclei were visualized using 4′, 6-diamidino-2-phenylindole
(DAPI, blue).
Egr1 has been well described as a participant in hippocampus-related
learning and memory. It plays an essential role in the maintenance
and formation of LTP, and adult neurogenesis as shown by its role in
the selection, maturation, and functional integration of dentate gyrus
newborn neurons [38]. Similar to our previous reported, the overexpression
of Egr1 was determined in the hippocampus at 12 weeks after
ovariectomy in our recent study. However, since there are also reports
showing downregulation of Egr1 in simian hippocampus, leading to
deficits in cognition [19], further studies on the determination of
Egr1 expression in different species and cells should be performed
to elucidate these discrepancies. Interestingly, our present study also
shown that when cognition deficiency occurs in mice 12 weeks after
ovariectomy, the levels of mTOR, phospho-mTOR and its downstream
targets, p70S6K and phospho-p70S6K are increased in hippocampus, in
agreement with some collected researches [39,40].
In the last decade, mTOR signaling has been extensively reported in
AD models, which demonstrated that the aberrant up-regulation of
mTOR signaling pathway might be associated with the development
of the neurodegenerative process [41,42]. To explore the relationship
between Egr1 and mTOR signaling pathway in OVX mice cognitive
impairment, OVX mice were treated with the mTOR inhibitor rapamycin.
In comparison to vehicle control, the rapamycin treatment negatively
regulated Egr1 mRNA and protein expression, in consistent with some
other studies [43,44].
Finally, we performed and verified this relationship between Egr1
gene and mTOR signaling pathway in SH-SY5Y cells. The subcellular
localization of Egr1 and mTOR was coexisting in SH-SY5Y cells. And
there was no alteration in the mTOR and p70S6K mRNA and protein
expression after transfection with the si-Egr1 fragment and the Egr1-
overexpressing plasmid. Consistent with the above results in hippocampus
of OVX mice, rapamycin treatment negatively regulated Egr1 mRNA and
protein expression, as well as mTOR/p70S6K signaling pathway.
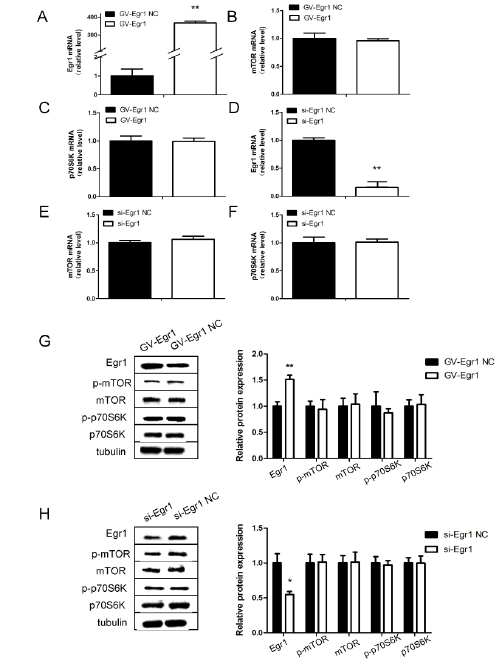
Figure 4: Expression of Egr1, mTOR and p70S6K in SH-SY5Y cells after transfection with Egr1-overexpressing plasmid or si-Egr1 fragment.
Cells were separately transfected with si-Egr1 control (si-Egr1 NC), si-Egr1 fragment (si-Egr1), GV141-Egr1 control (vector), GV141-Egr1 (GV141-
Egr1). Egr1 (A and D), mTOR(B and E) and p70S6K(C and F) messenger RNA levels in SH-SY5Y cells after transfection with Egr1-overexpressing
plasmid or si-Egr1 fragment. G, H: Relative protein levels of Egr1, mTOR and p70S6K after transfection. *P<0.05, **P<0.01.
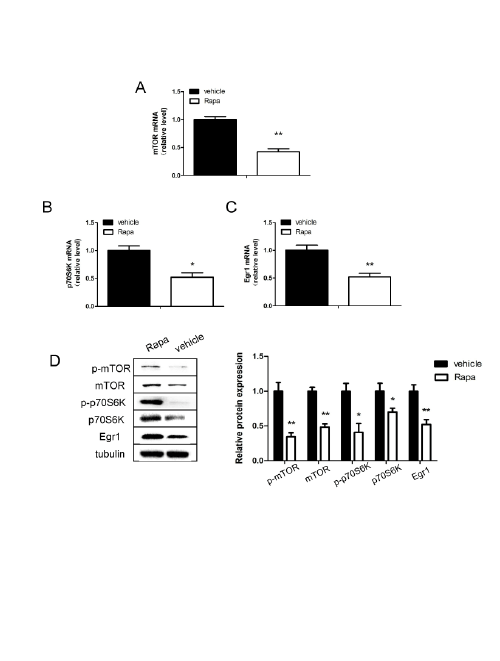
Figure 5: Expression of Egr1, mTOR and p70S6K in SH-SY5Y cells after treatment with rapamycin(4 µM).
mTOR (A), p70S6K (B) and Egr1 (C) messenger RNA levels in SH-SY5Y cells after treated with rapamycin for 48 hours. D: Relative protein levels
of Egr1, mTOR and p70S6K after treatment with rapamycin for 48 hours. *P<0.05, **P<0.01.
Taken together, our results suggest that the overexpression of Egr1 and
mTOR/p70S6K contributes to cognitive decline in OVX mice and Egr1
can be regulated by mTOR/p70S6K signaling in vivo and in vitro studies.
Egr1 maybe a downstream regulator of mTOR/p70S6K signaling pathway
in the pathogenesis of cognitive decline. The conclusion was similar to the
latest research indicating that Egr1 expression can be regulated by 4EBP1,
a classic downstream regulator of mTOR [45]. However, our further
studies are needed for a comprehensive understanding of mTOR/p70S6K/
Egr1 and molecular mechanisms of cognitive impairment in OVX model.
So far, there has not effective treatment or conventional drug for
cognitive impairment/AD in postmenopausal women. The signaling
network of cognition deficiency is complex, with many downstream
physiological outputs, and thus the mechanisms underlying its age-related
effects have not been fully elucidated. To our knowledge, this study is the
first to demonstrate that mTOR/p70S6K/ Egr1 signaling is involved in the
development and progress of cognitive dysfunction, which may play an
important role in pathogenesis of this disease and be a potential target in
a clinical practice of postmenopausal decline.
Conclusion
In summary, our data demonstrated that the over-expression of Egr1
and mTOR/p70S6K contributed to cognitive decline in OVX mice and
Egr1 could be regulated by mTOR/p70S6K signaling in vivo and in vitro
studies. The results of our current study showed that the mTOR/p70S6K/
Egr1 signaling was involved in the pathogenesis of cognitive dysfunction.
Our finding could provide insight into a novel mechanism of the
development and progression of postmenopausal decline in clinical practices.
Competing Interests
The authors declare that they have no conflict of interest.
Author’s Contributions
SZ: Experiments performance, Data Collection, Manuscript writing,
JC: Experiments performance, Data analysis, WZ: Data analysis, JW:
Project development, Manuscript editing.
Acknowledgment and Funding
We thank Ling Chen from department of physiology, Nanjing medical
university, for kindly providing the technical assistance of electro-
physiological examination. The study was supported by grant from
Chinese National Natural Science Foundation (No. 81170540), grant from
Jiangsu Province Science and Technology Commission for Natural Science
Foundation (No. BK2011846), and grant from Jiangsu Key Laboratory of
Neurodegeneration (No SJ11KF06) to Jie Wu.






