Abstract
Background: Hyperlipidemia is a condition in which blood lipid levels are greater than the normal range, and it is a major risk factor for the development of cardiac heart diseases. It affects 25-30% of the urban and 15-20% of the rural population. The current hyperlipidemia classification schemes and treatment levels are based on the Adult Treatment Program-3 guidelines issued by the National Cholesterol Education Panel, but there is no permanent treatment. So, the present study was aimed to investigate the antihyperlipidemic effect of Edaravone in Triton-X 100 and High Fat Diet-induced hyperlipidemic rat models.
Methods: The present study consists of two protocols. In the first protocol, animals were administered with Triton-X 100 (100 mg/kg, i.p. single dose), after 72 hours animals were divided into different groups (n=6) then treated with Edaravone (3 mg/kg, i.p. and 10 mg/kg, i.p.) as a test drug and Atorvastatin (10 mg/kg, p.o.) as a standard drug for 7 days. In the second protocol, High Fat Diet was administered for 8 weeks, then animals were divided into different groups (n=6), and treated with Edaravone (3 mg/kg, i.p. and 10 mg/kg, i.p.) as a test drug and Atorvastatin (10 mg/kg, p.o.) as a standard drug for last 2 weeks. At the end of experimentation, all rats were sacrificed, and then blood serum and liver were isolated for analysis of various biochemical parameters.
Results: Our results revealed that Edaravone shows protecting effect by lowering blood lipid levels (Total cholesterol, Triglyceride, low-density lipoprotein, very low-density lipoprotein, Total protein), and Atherogenic index, while increasing high-density lipoprotein. It also lowers the levels of liver enzymes (Serum Glutamic Oxaloacetate Transaminase, Serum Glutamate Pyruvate Transaminase, Alkaline phosphatase), average body weight, nitrites, and Malondialdehyde, while increasing the levels of antioxidants (superoxide dismutase, Catalase, Reduced Glutathione).
Conclusions: Our study findings suggest that Edaravone has antihyperlipidemic properties, as well as antioxidant activity.
Keywords
Edaravone; Antihyperlipidemic; High fat diet; Triton X-100; Atorvastatin; Antioxidant; Lipids; Liver enzymes
Abbreviations
AI: Atherogenic Index; ANOVA: Analysis of Variance; ALP: Alkaline Phosphatase; C: Cholesterol; CAD: Coronary Artery Disease; CAT: Catalase; CHD: Coronary Heart Disease; GSH: Reduced Glutathione; HDL: High-Density Lipoprotein; IAEC; Institutional Animal Ethics Committee; i.v: Intravenous; i.p.: Intraperitonial; LDL: Low-Density Lipoprotein; MI: Myocardial Infarction; MDA: Malondialdehyde; NCMH: National Commission on Macroeconomics and Health; PO: Per Oral; TC: Total Cholesterol; TG: Triglyceride; TCI: Tokyo Chemical Industry; TRI: Triton-X 100; HFD: High Fat Diet; SOD: Superoxide Dismutase; SEM: Standard Error Mean; SGOT: Serum Glutamic Oxaloacetate Transaminase; SGPT: Serum Glutamate Pyruvate Transaminase; TP: Total Protein; VLDL: Very Low-Density Lipoprotein; Ed: Edaravone
Introduction
Hyperlipidemia is a term used to describe a group of inherited and acquired diseases characterized by the high level of lipids in the human serum [1]. Lipids mainly include Total Cholesterol (TC), Triglyceride (TG), Low-Density Lipoprotein (LDL), Very-LowDensity Lipoprotein (VLDL), and High-Density Lipoprotein (HDL) [2]. Over three million persons in the United States and Europe today have hyperlipidemia, and the number is rapidly increasing. Patients with Coronary Artery Disease (CAD), occurring in males before the age of 55 to 60 years and females before the age of 65 years, had a high degree of hyperlipidemia. In the United States, cardiovascular disease is still the leading cause of morbidity and mortality. Recognized risk factors of hyperlipidemia are genetic susceptibility, familial hyperlipidemia, renal disorders, diabetes, and some drugs [3]. Endothelial damage, hyperlipidemia, inflammatory and immunologic variables, plaque erosion or rupture, hypertension, and smoking are all factors that contribute to the formation of atherosclerosis. Atherosclerosis develops as a result of underlying endothelial damage, which appears to be caused by nitric oxide depletion inside the endothelium [1]. Adults with exceptionally high LDL-C levels (190 mg/dL) are at an increased risk of Cardiovascular Disease (CVD) [4]. Hyperlipidemia directly affects cardiac electrophysiology and systolic function, which may be attributed to the progressive deposition of lipids and the resultant systemic oxidative stress, inflammatory response, and mitochondrial dysfunction. A high cholesterol level reduces coronary blood flow and causes capillary endothelial cell damage. It eventually leads to Left Ventricular Dysfunction (LV). It is proposed that hypercholesterolemia alters the membrane lipid bilayer, regulates intracellular calcium ions, and makes the myocardium more vulnerable to external injuries such as myocardial ischemia, hemodynamic overload, and diabetes [5]. While statins are the most often used lipid-lowering medications, their beneficial benefits on the heart go far beyond decreasing blood lipids [6]. In statin-tolerant individuals, however, omega-3 fatty acids, fibrates, and niacin are routinely employed as therapeutic choices. Previous research has demonstrated that HMG-CoA is an essential enzyme required in cholesterol-related pathways. In addition, mevalonate also has some physiological role in other pathways [7]. Furthermore, investigating the pharmacological effects of lipid-lowering medicines on the heart may reveal valuable insights into the processes by which hyperlipidemia affects the heart directly. A recent large-scale investigation revealed that lipid-lowering medicine can minimize the risk of cardiovascular events. Fibrates are fibric acid derivatives that have been used to treat hypertriglyceridemia by lowering blood triglyceride levels [8]. People with hyperlipidemia have no evident indications or symptoms, but heart structure and function may have started to decline. As a result, strong detection approaches should be developed to detect these alterations at an early stage [5]. LDL-C circulation is linked to atherosclerotic plaques, whereas low LDL-C circulation prevents the development of atherosclerosis. However, HDL particles play an important role in the anti-atherosclerotic action by accepting cholesterol and transporting it to the liver. HDL has an anti-oxidative role inside the body and it inhibits the development of atheroma in the sub-endothelial area [9]. A reduction in antioxidant capacity inside the body is responsible for the generation of reactive Oxygen Species (ROS), which leads to endothelial dysfunction, dyslipidemia, and atherosclerosis [10]. In many clinical diseases, free radicals play important roles in the development of tissue damage, and removing free radicals may offer a novel therapeutic option.
Edaravone also konwn as MCI-186, its chemical name is 3-methyl1-phenyl-2-pyrazoline-5-one. Edaravone is a powerful free-radical scavenger that has been on the market for more than 30 years in Japan by the Mitsubishi Tanabe Pharma. Since 2001, it has been used to treat strokes. Aside from anti-oxidant properties, Edaravone has been shown to improve antioxidant levels, nitric oxide generation, and apoptotic cell death. The FDA has authorized Edaravone as a therapy for Amyotrophic Lateral Sclerosis (ALS) patients. Medication repurposing is a novel strategy for drug development in which pharmaceuticals are identified for uses other than their original indication [11]. It has recently been demonstrated that Edaravone may diffuse into numerous organs and that it regulates inflammatory processes, elimination of lipid peroxides, nitric oxide generation, ROS generation, and apoptotic cell death, in addition to its effects on hydroxyl radical elimination. Edaravone also protects against various animal models of tissue damages, including lung, intestinal, myocardial, pancreatic, liver, renal injury, and other diseases in various experimental studies [12]. Furthermore, Edaravone has been shown to reduce myocardial damage after ischemia and reperfusion in individuals with acute myocardial infarction [13].
Because these illnesses are caused by oxidative stress or induction of cytokine, Edaravone may provide a novel therapeutic option for endothelial dysfunction in the context of atherosclerosis, heart failure, diabetes, or hypertension [14]. It offers a novel therapeutic strategy for treating cardiovascular disorders. In this research, we looked at the biological effects of Edaravone on rats’ blood lipid levels, liver enzymes, and endogenous antioxidants. We hypothesized that Edaravone would be useful in lowering blood lipid levels and producing an antioxidant impact. This discovery opens new opportunities for the treatment of hyperlipidemia.
Material and Methods
Chemicals and drugs
Analytical-grade chemicals were utilized in this study. Atorvastatin was procured from Macleods Pharmaceuticals Ltd., Surat, Gujrat, India, as a gift sample. Edaravone was purchased from Sigma Aldrich and Triton-X 100 was purchased from Hi-Media. Other chemicals and reagents like sodium potassium tartrate, Copper sulfate, sodium hydroxide, sodium carbonate, bovine serum albumin, folin phenol reagent, dipotassium phosphate, monopotassium phosphate, hydrogen peroxide, tetrasodium pyrophosphate, hydrochloric acid, Phenazone Methosulphate (PMS), Nicotinamide Adenine Dinucleotide Reduced (NADH), Nitroblue Tetrazolium (NBT), glacial acetic acid, n-butanol, sodium phosphate monobasic, Ethylenediaminetetraacetic Acid (EDTA), sodium azide, glutathione, trichloroacetic acid, Dithiobisnitrobenzoic Acid (DTNB), thiobarbituric acid were acquired from central drug stock of SLT Institute of Pharmaceutical Sciences, GGV, Bilaspur (C.G).
For estimation of the biochemical parameter; Diagnostic kits like HDL, LDL, TG, TC, enzymes Serum Glutamic Oxaloacetate Transaminase (SGOT/AST), Alkaline Phosphate (ALP), Total Protein (TP) were obtained from Beacon Diagnostics Pvt. Ltd., India. A plasma Serum Glutamic Pyruvic Transaminase (SGPT) kit was obtained from Viola Diagnostic Systems, India. A plasma (ALP) kit was obtained from AGD Biomedicals (P) Ltd., Mumbai.
Animals
Wistar albino rats (150-200 g) of either sex were purchased from Chakraborty enterprises, Kolkata -700011 Reg. no.-994/GO/Re/S/06/ CPCSEA). Rats were kept in the animal house of GGV, Bilaspur. Rats were acclimatized for 7 days before experimentation with standard situations of relative humidity 45-55%, room temperature 25 ± 10C following 12h of the light-dark cycle.
Rats were allowed free access to rat pellet (Kapila Posuahar) and water provided ad libitum in the strict hygienic environments. The study design was approved by the Institutional Animal Ethics Committee (IAEC) of the Institute of Pharmaceutical Sciences, Guru Ghasidas Vishwavidyalaya, Bilaspur, (280/IAEC/Pharmacy/2020).
Experimental design
Triton X-100 induced animal model: In this model, hyperlipidemia was induced in animals by single-dose administration of Triton X-100 (100 mg/kg, i.p.) [15]. Wistar albino rats (either sex; 150-200 g) were randomly selected and divided into five groups (n=6/group). Normal group (group 1) received 5% DMSO (5 ml/kg/day i.p.). Remaining four groups sensitized with Triton X-100 (100 mg/kg, i.p.), 72 hours before the administration of Edaravone and Atorvastatin. Thereafter, Triton X-100 control group received DMSO (5 ml/kg/day, i.p.), standard group received Atorvastatin (10 mg/kg/day, p.o.), Ed-1 group received Edaravone (3 mg/kg/day, i.p.), and Ed-2 group received Edaravone (10 mg/kg/day, i.p.) for concecutive 7 days [16,17] (Table 1).
| S.No. |
Group |
Treatment |
| 1 |
Normal |
Standard diet + 5% DMSO v/v (5 ml/kg) for 7 days |
| 2 |
Triton X-100 control |
Triton (100 mg/kg i.p.) + 5% DMSO for 7 days |
| 3 |
STD |
Triton (100 mg/kg i.p.) + Atorvastatin (10 mg/kg/ day p.o.) for 7 days |
| 4 |
Ed-1 |
Triton (100 mg/kg i.p.) + Edaravone (3 mg/kg/day i.p.) for 7 days |
| 5 |
Ed-2 |
Triton (100 mg/kg i.p.) + Edaravone (10 mg/kg/ day i.p.) for 7 days |
Table 1: Triton X-100 induced animal model.
High-Fat-Diet induced (HFD) animal model: Wistar albino rats (either sex; 150-200 g) were randomly selected and divided into five groups (n=6/group. Animals of the normal group were fed standard diet entire the experimental protocols and after 6 weeks, treated with 5% DMSO (5 ml/kg/day, i.p.) for the next 2 weeks. Hyperlipidemia was induced in rest groups by chronic administration of HFD containing 68% powdered standard pellets, 30% dalda, and 2% cholesterol, cholic acid, sugar, butter, and casein for 6 weeks. After that for next 2 weeks, HFD control group received 5% DMSO (5 ml/kg/day, i.p.), standard group received Atorvastatin (10 mg/kg/day, p.o.), Ed-1 group received Edaravone (3 mg/kg/day, i.p.), and Ed-2 group received Edaravone (10 mg/kg/day, i.p.), concurrently with HFD (Table 2).
| S.No. |
Group |
Treatment |
| 1 |
Normal |
Standard diet + 5% DMSO v/v for 2 weeks |
| 2 |
Triton X-100 control |
HFD upto 8 weeks + 5% DMSO for 2 weeks |
| 3 |
STD |
HFD upto 8 weeks + Atorvastatin (10 mg/kg p.o.) for 2 weeks |
| 4 |
Ed-1 |
HFD upto 8 weeks + Edaravone (3 mg/kg i.p.) for 2 weeks |
| 5 |
Ed-2 |
HFD upto 8 weeks + Edaravone (10 mg/kg i.p.) for 2 weeks |
Table 2: HFD induced animal model.
Collection of blood and preparation of tissue homogenate: Blood samples were collected through cardiac puncture and serum was separated via centrifugation for 10 minutes at 3000 rpm. Plasma was isolated and stored at −20°C till analysis. For the determination of oxidative stress markers, liver tissue was isolated and kept in pH 7.4 neutral buffer formalin and homogenized with phosphate buffer pH 7.4 and centrifuged for 15 min at 8000 rpm. The supernatant was collected and consumed for the assessment of several biochemical parameters.
Biochemical assessment
Serum lipid profile and hepatic enzyme assessment: Serum TC, TG, HDL, SGOT, ALP, TP was estimated by the procedure outlined in the commercial diagnostic kit, and SGPT was estimated by a commercial kit.
Serum LDL, and VLDL, is calculated by Friedewald WT, et al. LDLC=TC-(HDL-C+VLDL-C), VLDL C=Triglyceride/5 [18]. Atherogenic Index (AI) was estimated by the formula AI=(Total cholesterolHDL-C)/HDL-C [19].
Estimation of antioxidant, oxidative stress biomarker and nitrite content: Catalase (CAT) was analyzed by the process of Aebi H [20] Superoxide Dismutase (SOD) was analyzed by the procedure described in Kakkar P, et al. [21]. Reduced Glutathione (GSH) was analyzed by the process of Ellman GL [22]. Malondialdehyde (MDA)/ Lipid Peroxidation (LPO) were estimated through a spectrophotometer by assessing Thiobarbituric Acid Reactive Substances (TBARS) by the method of Goyal R, et al. [23]. Nitrite content was determined by the procedure described in Guevara I, et al. [24].
Statistical analysis
Outcomes of six observations were stated as mean ± S.E.M. Statistical evaluation was performed by using Graph pad prism version 5.0 software. The distinctions between numerous groups were statically examined by using a one-way analysis of variance, and Twoway Analysis of Variance (ANOVA) chased by a post hoc test. Values of P<0.05 were considered statistically significant.
Results
Effect of Edaravone on serum lipids level
Results of the serum lipids level in hyperlipidemic control groups (triton X-100 and HFD control group), as compared to the normal group, a significant (P<0.05) increase in serum TC, TG, LDL, and VLDL levels and decreased in HDL level were observed. In the Edaravone treated group (Ed-1 and Ed-2) and standard group, as compared to the hyperlipidemic control groups, significant (P<0.05) restoration in serum TC, TG, LDL, VLDL, and HDL levels were observed. Results show that Edaravone considerably alleviates the hyperlipidemic condition against triton X-100 and HFD treatment (Tables 3 and 4).
| S.No. |
Group |
TC
(mg/dl) |
TG
(mg/dl) |
HDL
(mg/dl) |
LDL
(mg/dl) |
VLDL
(mg/dl) |
| 1 |
Normal |
184.2 ± 15.83 |
126.1 ± 6.24 |
66.14 ± 4.89 |
110.3 ± 6.55 |
25.99 ± 2.97 |
| 2 |
Triton X-100 control |
410.6 ± 9.56# |
167.4 ± 9.49# |
41.88 ± 2.13# |
336.5 ± 10.17# |
33.48 ± 4.40# |
| 3 |
STD |
216.9 ± 10.66* |
132.4 ± 9.50* |
65.45 ± 2.31* |
125.3 ± 4.91* |
26.04 ± 3.43* |
| 4 |
Ed-1 |
355.2 ± 12.56* |
159.0 ± 4.58* |
50.37 ± 5.23* |
274.5 ± 10.11* |
31.22 ± 2.10* |
| 5 |
Ed-2 |
316.7 ± 6.53* |
133.1 ± 10.01* |
68.53 ± 0.60* |
222.8 ± 9.73* |
26.94 ± 2.87* |
Table 3: Effect of Edaravone on serum lipid profile in triton X-100 induced hyperlipidemic rats.
Values are expressed as mean ± SEM (n=6), statistical evaluation was carried out using one-way analysis of variances followed by Tukey’s post hoc test.
# P<0.05 when compared to the normal group and * P<0.05 when compared to the triton X-100 control group.
|
S.No. |
Group |
TC
(mg/dl) |
TG
(mg/dl) |
HDL
(mg/dl) |
LDL
(mg/dl) |
VLDL
(mg/dl) |
| 1 |
Normal |
194.8 ± 9.86 |
141.5 ± 1.13 |
78.81 ± 5.95 |
99.64 ± 6.09 |
28.30 ± 0.22 |
| 2 |
HFD control |
450.6 ± 10.9# |
286.2 ± 3.77# |
43.80 ± 4.53# |
350.4 ± 10.26# |
57.25 ± 0.75# |
| 3 |
STD |
230.5 ± 10.04* |
186.7 ± 1.19* |
72.80 ± 5.39* |
121.6 ± 9.79* |
37.34 ± 0.23* |
| 4 |
Ed-1 |
370.6 ± 21.53* |
216.6 ± 0.88* |
55.47 ± 6.59* |
272.7 ± 10.27* |
43.33 ± 0.17* |
| 5 |
Ed-2 |
329.6 ± 7.22* |
158.8 ± 1.17* |
61.97 ± 4.60* |
327.7 ± 9.99* |
31.75 ± 0.23* |
Table 4: Effect of Edaravone on serum lipid profile in high fat diet induced hyperlipidemic rats.
Values are expressed as mean ± SEM (n=6), statistical evaluation was carried out using one-way analysis of variances followed by Tukey’s post hoc test.
# P<0.05 when compared to the normal group and * P<0.05 when compared to the HFD control group.
Effect of Edaravone on atherogenic index
Results showed a considerable increase in AI in hyperlipidemic control groups (triton X-100 and HFD control group). In hyperlipidemic control groups (triton X-100 and HFD control group), as compared to the normal group, a significant (P<0.05) increase in AI was observed. Whereas, in the Edaravone treated group (Ed-1 and Ed-2) and standard group, as compared to the hyperlipidemic control groups, a significant (P<0.05) reduction in AI was observed. The Results indicate that Edaravone treatment decreases the atherosclerotic events against hyperlipidemia (Figure 1).
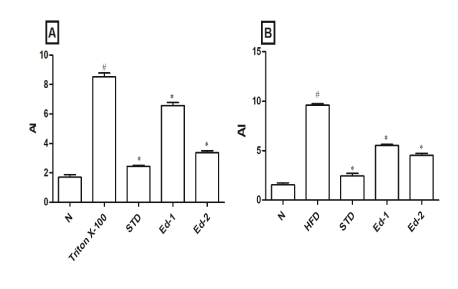
Figure 1: Effect of Edaravone on atherogenic index in (A) triton X-100 and (B) high fat diet induced animal model. Values are expressed as mean ± SEM (n=6), statistical evaluation was carried out using one-way analysis of variances followed by Tukey’s post hoc test. # P<0.05 when compared to the normal group and * P<0.05 when compared to the triton X-100 or HFD control group.
Effect of Edaravone on serum hepatic enzymes (SGOT, SGPT, and ALP) and total protein contents
Results show that Edaravone considerably alleviates the hepatic enzymes against triton X-100 and HFD treatment. In hyperlipidemic control groups (triton X-100 and HFD control group), as compared to the normal group, a significant (P<0.05) increase in serum SGOT, SGPT, ALP, and TP level were observed. In the Edaravone treated group (Ed-1 and Ed-2) and standard group, as compared to the hyperlipidemic control groups, significant (P<0.05) restoration in an increase in serum hepatic SGOT, SGPT, ALP, and TP were observed (Tables 5 and 6).
| S.No. |
GROUPS |
SGOT (U/L) |
SGPT (U/L) |
ALP (U/L) |
TP(g/dL) |
| 1 |
Normal |
30.45 ± 2.25 |
24.42 ± 4.07 |
45.86 ± 1.19 |
5.29 ± 0.15 |
| 2 |
Triton X-100 control |
72.84 ± 5.40# |
83.14 ± 9.72# |
165.4 ± 7.65# |
8.23 ± 0.65# |
| 3 |
STD |
28.76 ± 1.91* |
22.89 ± 2.19* |
51.56 ± 0.70* |
5.23 ± 0.37* |
| 4 |
Ed-1 |
39.03 ± 2.12* |
44.61 ± 3.96* |
74.61 ± 0.35* |
6.34 ± 0.96* |
| 5 |
Ed-2 |
30.59 ± 1.18* |
33.35 ± 2.74* |
55.58 ± 0.40* |
6.00 ± 0.08* |
Table 5: Effect of Edaravone on hepatic enzymes and total protein level in triton X-100 induced hyperlipidemic rats.
Values are expressed as mean ± SEM (n=6), statistical evaluation was carried out using one-way analysis of variances followed by Tukey’s post hoc test. # P<0.05 when compared to the normal group and * P<0.05 when compared to the Triton X-100 control group.
|
S.No. |
GROUPS |
SGOT (U/L) |
SGPT (U/L) |
ALP (U/L) |
TP(g/dL) |
| 1 |
Normal |
27.70 ± 1.02 |
21.94 ± 1.27 |
66.11 ± 5.22 |
5.57 ± 0.56 |
| 2 |
HFD control |
62.00 ± 4.78# |
62.20 ± 0.78# |
150.7 ± 4.92# |
8.39 ± 1.18# |
| 3 |
STD |
27.42 ± 1.15* |
23.52 ± 1.89* |
75.48 ± 4.38* |
6.54 ± 0.62* |
| 4 |
Ed-1 |
35.61 ± 1.02* |
48.23 ± 3.89* |
105.9 ± 4.30* |
6.82 ± 0.24* |
| 5 |
Ed-2 |
33.17 ± 5.57* |
34.57 ± 2.34* |
82.79 ± 4.93* |
6.42 ± 0.21* |
Table 6: Effect of Edaravone on hepatic enzymes and total protein level in high fat diet induced hyperlipidemic rats.
Values are expressed as mean ± SEM (n=6), statistical evaluation was carried out using one-way analysis of variances followed by Tukey’s post hoc test.
# P<0.05 when compared to the normal group and * P<0.05 when compared to the HFD control group.
Effect of Edaravone on oxidative stress biomarkers
Results of the oxidative stress biomarkers in liver tissues are estimated. In hyperlipidemic control groups (triton X-100 and HFD control group), as compared to the normal group, a significant (P<0.05) decrease in antioxidants (SOD, CAT, and GSH) were observed. In addition, hyperlipidemia control groups showed a significant increase in MDA and nitrite content as compared to the normal group. In the Edaravone treated group (Ed-1 and Ed-2) and standard group, as compared to the hyperlipidemic control groups, significant (P<0.05) restoration in the antioxidants, MDA, and nitrite content were observed. Results indicate the potential antioxidant property of Edaravone (Tables 7 and 8).
| S.No. |
Groups |
SOD(Units/mg) |
CAT(Units/mg) |
GSH (nmoles/mg) |
MDA (nmoles/mg) |
Nitrite (nmoles/mg) |
| 1 |
Normal |
0.61 ± 0.08 |
3.68 ± 0.30 |
3446 ± 188.5 |
460.6 ± 16.97 |
0.47 ± 0.02 |
| 2 |
Triton X-100 control |
0.39 ± 0.07# |
1.15 ± 1.03# |
1865 ± 91.75# |
772.3 ± 59.52# |
2.36 ± 0.27# |
| 3 |
STD |
0.56 ± 0.07* |
3.61 ± 0.29* |
3155 ± 85.52* |
466.6 ± 16.15* |
0.78 ± 0.08* |
| 4 |
Ed-1 |
0.48 ± 0.06* |
2.36 ± 0.26* |
2794 ± 34.38* |
414.9 ± 6.640* |
1.34 ± 0.11* |
| 5 |
Ed-2 |
0.55 ± 0.04* |
3.67 ± 0.36* |
3069 ± 89.41* |
463.9 ± 18.93* |
0.59 ± 0.09* |
Table 7: Effect of Edaravone on hepatic oxidative stress biomarkers in triton X-100 induced hyperlipidemic rats.
Values are expressed as mean ± SEM (n=6), statistical evaluation was carried out using one-way analysis of variances followed by Tukey’s post hoc test.
# P<0.05 when compared to the normal group and * P<0.05 when compared to the Triton X-100 control group.
| S.No. |
Groups |
SOD
(Units/mg) |
CAT
(Units/mg) |
GSH (nmoles/mg) |
MDA
(nmoles/mg) |
Nitrite (nmoles/mg) |
| 1 |
Normal |
0.91 ± 0.09 |
5.63 ± 0.17 |
2220 ± 93.05 |
474.8 ± 10.47 |
0.48 ± 0.04 |
| 2 |
HFD control |
0.60 ± 0.11* |
1.93 ± 0.97* |
1217 ± 75.04* |
720.1 ± 7.720* |
2.85 ± 0.20# |
| 3 |
STD |
0.87 ± 0.12* |
5.50 ± 0.14* |
2237 ± 53.69* |
523.6 ± 15.88* |
0.88 ± 0.10* |
| 4 |
Ed-1 |
0.71 ± 0.12* |
5.20 ± 0.99* |
1862 ± 106.2* |
646.6 ± 32.74* |
1.29 ± 0.14* |
| 5 |
Ed-2 |
0.84 ± 0.11* |
5.51 ± 0.11* |
2349 ± 64.36* |
538.8 ± 35.76* |
0.77 ± 0.09* |
Table 8: Effect of Edaravone on hepatic oxidative stress biomarkers in high fat diet induced hyperlipidemic rats.
Values are expressed as mean ± SEM (n=6), statistical evaluation was carried out using one-way analysis of variances followed by Tukey’s post hoc test.
# P<0.05 when compared to the normal group and * P<0.05 when compared to the HFD control group.
Effects of Edaravone on average body weight of HFD treated hyperlipidemic rats
Results on average body weight gain. The chronic fed of a highfat diet in high-fat diet treated groups (HFD control, standard, Ed-1, and Ed-2 group) led to a significant (P<0.05) increase in average body weight in time dependant manner from the second week to the sixth week as compared with the normal group. After six weeks, for the next 2 weeks, Edaravone and Atorvastatin treatments in their respective groups led to a significant (P<0.05) reduction in average body weight gain as compared with the HFD control group (Figure 2).
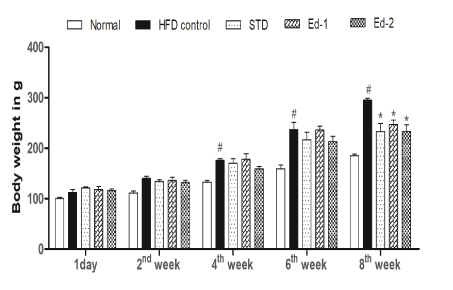
Figure 2: Effect of Edaravone on average body weight gain in high fat diet induced hyperlipidemic rats. Values are expressed as mean ± SEM (n=6), statistical evaluation was carried out using one-way analysis of variances followed by Bonferroni’s post hoc test. # P<0.05 when compared to the normal group and * P<0.05 when compared to the HFD control group.
Discussion and Conclusion
Hyperlipidemia and atherosclerosis are the chief contributors to the pathogenesis of cardiovascular. Literature has shown that atherosclerosis may be affected by obesity through unrecognized variables and risk factors for coronary artery disease, like dyslipidemia, glucose intolerance, hypertension, inflammatory markers, the prothrombotic state, and obesity is the major component, which contributes to the overall burden of cardiovascular diseases worldwide [25]. Cardiac diseases are the prominent reason for worldwide mortality, which accounts for approximately 17 million demises per annum [26].
The current research findings show that both experimental models showed significant induction in hyperlipidemia that is indicated by significant elevation of serum TC, TG, LDL, and VLDL and reduction of serum HDL. Triton X-100 is a surfactant that blocks the elimination of TG-rich lipoprotein by inhibiting the lipoprotein lipases and uptake of lipoproteins from the circulation via extrahepatic tissues and produced acute hyperlipidemia [27]. In addition, Diet habits have a significant role in cholesterol metabolism. It is established that HFD induces hyperlipidemia by altering cholesterol absorption and metabolism [28]. In the current study, we observed that the Edaravone treatment at 3 mg/kg and 10 mg/kg dose levels, significantly restore the serum lipid profile against hyperlipidemia. Results also show that Edaravone at 10 mg/kg dose had better effects than 3 mg/kg dose.
It is well known that elevated TC, TG and LDL, and decreased HDL levels are related to an augmented risk of atherosclerosis and CHD [29]. The risk of atherosclerosis was measured by AI, results show that the hyperlipidemic control group significantly increased the AI, which was considerably decreased by Edaravone treatments. These results indicate the potential beneficial antihyperlipidemic effects of Edaravone.
Moreover, Edaravone treatment restores the serum hepatic enzymelike SGOT, SGPT, ALP, and serum TP contents against hyperlipidemia. It is well established that serum SGOT, SGPT, and ALP are authentic markers of liver function. An increase in the activities of SGOT, SGPT, and ALP is might be due to leakage of these enzymes from the liver cytosol into the bloodstream. Which indicates hepatic damage [30]. Additionally, the increased serum TP content also demonstrated tissue damage [31]. The restoration of such serum enzyme and total protein contents, indicating functional improvement of hepatocytes.
Oxidative stress is another biomarker for tissue damages. It is well accepted that oxidative stress leads to oxidative damages of the tissues and exacerbates the condition of the disease [32]. Oxidative stress is a condition, their antioxidant defense system that is disrupted due to excess production of Reactive Oxygen Species (ROS) and/or depletion of antioxidants. The elevation of ROS formation further leads to oxidative damage on cellular protein and lipids. Various endogenous enzymatic like CAT and SOD and nonenzymatic antioxidants (GSH) are engaged in the aerobic cells to mitigate the oxidative damaging effect of ROS [33]. The reduction of such antioxidants indicates the oxidative stress state, which was observed in the hyperlipidemic control group. Moreover, increased MDA and nitrite content are other important parameter to determine oxidative stress [34], which was observed in, hyperlipidemic control group. The results of the present study showed that treatment of Edaravone alleviated oxidative stress by elevating the levels of hepatic antioxidants (CAT, SOD, and GSH) and lowering the level of MDA and nitrite contents. These effects show the potential antioxidant properties of Edaravone, which was also established in previous studies [35]. The study has a lack of explanation of the specific mechanism of action of Edaravone but based on the finding we concluded that the antihyperlipidemic and antiatherosclerosis effects of Edaravone are might be due to its potent antioxidant properties.
Conflict of Interest
None.
Authors Contribution
Both the authors contributed equally.
Funding
None.



